Infrared (IR)
Comprehensive IR spectroscopy reveals molecular detail of active organocatalyst
Jun 24 2025
Catching reactive intermediates of catalysed reactions
Researchers from the University of Amsterdam and the HFML-FELIX Institute for Matter and Energy in Nijmegen have used infrared spectroscopy to examine the structural behaviour of a thiourea-based organocatalyst during a model reaction. The study provides molecular-level data on how the catalyst binds to a reactant and changes conformation during the process. Thiourea – SC(NH₂)₂ – is an organosulphur compound and is structurally similar to urea – OC(NH₂)₂ – but the urea’s oxygen atom has been replaced by a sulphur atom to make thiourea.
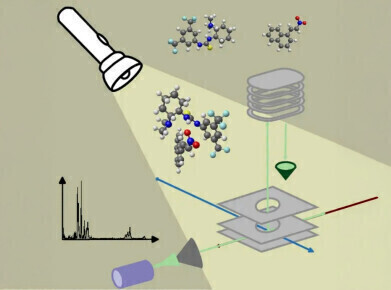
The work, led by Professor Wybren Jan Buma of Molecular Photonics, combined the tunable infrared radiation of the FELIX free-electron laser with molecular beam techniques and quantum chemical calculations. This approach enabled the characterisation of both the unbound catalyst and the catalyst–reactant complex, capturing vibrational features associated with binding and hydrogen bonding.
The focus of the study was ‘Takemoto’s catalyst’, a compound often used as a benchmark for investigating hydrogen-bond-driven organocatalysis. Organocatalysts, which are small, metal-free molecules, are being increasingly studied as alternatives to metal-based catalysts due to concerns about cost, toxicity and environmental impact. Many also offer good control over stereochemistry, a vital consideration in pharmaceutical development.
Using a molecular beam to isolate catalyst–reactant pairs in the gas phase, the researchers probed their structure by directing FELIX’s infrared light (covering 650–3,500 cm⁻¹) through the beam. This allowed detection of vibrational signatures linked to both the catalyst and the catalyst–reactant complex. Quantum chemical modelling helped assign these spectral features to specific structural motifs and hydrogen-bonding interactions.
One of the key observations was that the catalyst must adopt a different conformation when engaging with the reactant. Although this geometry is not favoured under equilibrium conditions, it can be stabilised briefly during the rapid cooling that occurs in the expanding molecular beam. This allowed the team to record the vibrational spectrum of the reactive intermediate.
While earlier spectroscopic studies of catalytic intermediates have largely focused on ionic species, this research extends the approach to neutral molecules, which are more difficult to study under standard conditions. The method could be applicable to a broader set of catalytic systems and may support ongoing efforts to understand and improve catalyst performance through structural design.
The findings are reported in The Journal of Physical Chemistry Letters.
For further reading please visit: 10.1021/acs.jpclett.5c01093
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh