IT solutions
PerkinElmer Syngistix for ICP Enhanced Security for 21 CFR Part 11 Compliance
Nov 07 2018
For pharmaceutical companies and their suppliers to be able to sell products into the United States, it is mandatory to comply with 21 CFR Part 11 regulations, which include criteria around electronic records, electronic signatures, and audit trails to ensure data integrity and reliability during the analytical testing. If your laboratory needs to test for elemental impurities in pharmaceutical products, trust PerkinElmer to provide the software functionality to fully meet these technical requirements. Introducing the NEW Syngistix™ for ICP Enhanced Security™ software version 4.0 for PerkinElmer ICP instruments, with key security features including:
- Secure audit trail
- Electronic signatures and records
- Data integrity
- Password-protected database file structure
- Login history
- Configurable password control
Not only does the software provide the required functionality to aid in compliance with 21 CFR Part 11, it does so simply:
- 1. Administrators define users & groups, set up permissions, electronic signatures, and security settings – and much more under User Setup, a simple but powerful app outside of the main Syngistix operating environment.
- 2. Once user permissions and e-signature parameters are set and samples are ready, the operator can perform analyses within the limitations defined by the Administrator in User Setup, guaranteeing traceability and data integrity. And while samples are running, it’s easy to switch between Users so that, for example, a Reviewer can review previously run samples without having to wait for the current set of samples to finish. If you don’t want anybody touching the software during an analysis, the software can be locked with a simple click.
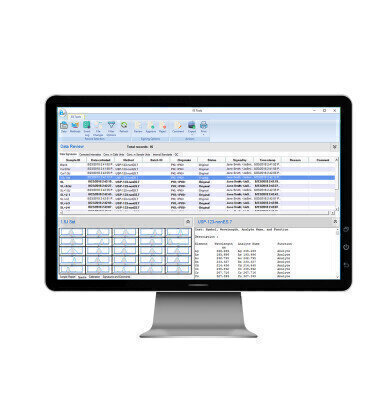
- 3. After an analysis is complete, the data can be quickly reviewed and approved/rejected through ES Tools. This dashboard interface lets you quickly and easily review data, methods, the audit trail, event logs, and file changes. With the ability to filter, sort, and compare entries, it’s easy to find what you’re looking for. Reviewing, approving, rejecting, and adding comments are just a click away, along with the ability to export and print individual samples and batches in a human readable format.
Syngistix for ICP Enhanced Security software version 4.0 provides regulated laboratories powerful but simple-to-use tools to meet the technical requirements for 21 CFR Part 11 compliance, offering flexibility while providing electronic signature points, maintaining data integrity through a database file structure, and implementing a secure audit trail.
Learn more about the new capabilities of Syngistix for ICP Enhanced Security software - download our product and application resources.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh