News
Stem cell therapy approved by EMA for treatment of patients with blood cancers
Jul 01 2025
The European Medicines Agency (EMA) has recommended granting a conditional marketing authorisation in the European Union (EU) for Zemcelpro (dorocubicel / unexpanded umbilical cord cells) for the treatment of adults with haematological malignancies. Zemcelpro is intended for patients requiring an allogeneic haematopoietic stem cell transplantation (allo-HSCT) following myeloablative conditioning, where no suitable donor cells are otherwise available.
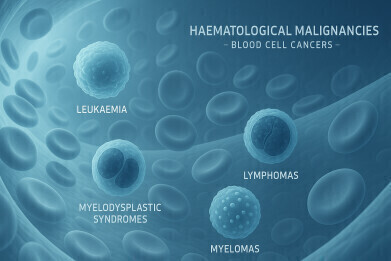
Haematological malignancies – blood cell cancers – include leukaemia, lymphomas, myelodysplastic syndromes and myelomas. These conditions are commonly diagnosed and allo-HSCT remains the only potentially curative treatment in some cases. This approach involves replacing the patient’s bone marrow with healthy stem cells from a donor, enabling the formation of new marrow and the production of normal blood cells.
While stem cells are typically sourced from matched donors, such as siblings or unrelated individuals, umbilical cord blood provides an alternative when matched donors are unavailable. However, the limited cell count in cord blood can delay engraftment. Zemcelpro addresses this limitation by combining unexpanded and expanded (dorocubicel) cord blood stem cells to enhance efficacy.
Developed by Cordex Biologics International Limited, Zemcelpro received eligibility to the EMA’s PRIority MEdicines (PRIME) scheme in December 2020 following designation as an orphan medicinal product in April 2020. The therapy’s approval was based on pooled data from two open-label, single-arm studies which involved 25 patients. Among these, 84% achieved neutrophil engraftment within a median of 20 days, while 68% reached platelet engraftment within a median of 40 days.
Across a broader safety population of 116 patients, the most frequently reported adverse effects included cytopenia – the reduction in the number of mature blood cells in circulation – infection, hypertension and engraftment syndrome. Acute graft-versus-host disease (GvHD) occurred in 60% of patients within the first 100 days post-transplant, while chronic GvHD was seen in 13% of cases within one year. Details on mitigation strategies are outlined in the product’s information leaflet and risk management plan.
Following a scientific review, the Committee for Advanced Therapies (CAT) determined that the benefits of Zemcelpro outweighed its associated risks for patients lacking access to a matched donor. The Committee for Medicinal Products for Human Use (CHMP) endorsed this conclusion and issued a positive opinion recommending conditional approval.
Conditional marketing authorisation enables earlier patient access to therapies addressing unmet medical needs, based on less comprehensive data than normally required. The company has been asked to provide long-term results from the initial studies, conduct a randomised controlled trial and undertake a registry-based study to further confirm Zemcelpro’s safety and efficacy.
The CHMP’s opinion now passes to the European Commission for a final decision on authorisation. If approved, pricing and reimbursement decisions will be determined by individual EU Member States, in line with their respective healthcare systems.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh