Research news
Calibr–Skaggs doses first patient with switchable CAR-T cell therapy in phase 1 trial for metastatic breast cancer
Jun 25 2025
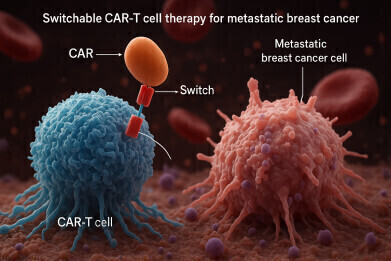
CLBR001 + ABBV-461 is a novel investigational two-part switchable chimeric antigen receptor T cell (sCAR-T) immunotherapy designed to treat solid tumours
The Calibr–Skaggs Institute for Innovative Medicines, the drug development division of Scripps Research, has announced the dosing of the first patient in a phase 1 clinical trial (NCT06878248) investigating CLBR001 + ABBV-461, a modular, switchable chimeric antigen receptor T cell (sCAR-T) therapy for patients with advanced or metastatic breast cancer who lack suitable treatment options. This marks the first clinical study of the sCAR-T platform in solid tumours.
CAR-T cell therapy has significantly advanced treatment outcomes for patients with haematological malignancies who have relapsed after multiple prior therapies. However, its success in solid tumours has been limited. Calibr–Skaggs’ switchable CAR-T platform aims to overcome the biological barriers that have so far restricted the efficacy of traditional CAR-T approaches in solid tumours.
The sCAR-T platform developed at Calibr–Skaggs is designed to improve both the precision and safety of CAR-T therapies. It has already shown encouraging preliminary results in a phase 1 trial for haematological malignancies. Early data indicate that CLBR001 cells can expand more robustly in the body than conventional CAR-T cells, offering a potentially improved capacity to infiltrate the immunosuppressive solid tumour microenvironment. Moreover, the addition of the switch mechanism may reduce the risk of T cell exhaustion by allowing the therapy to be paused or modulated.
“There is a critical need to develop gene and cell therapies that can reproduce the remarkable success observed in blood cancers for patients with solid tumours such as breast cancer,” said Dr Travis Young, Vice President of Biology at Calibr–Skaggs.
“By integrating an antibody-based ‘switch’, we hope to increase the targeting precision of solid tumour cells while addressing safety concerns,” he added.
Researchers engineer herpes virus to enhance T cell activity for cancer immunotherapy
The technique could be developed into a novel oncology treatment vectorA team at the University of Michigan (U-M) has developed a novel approach to cancer immunotherapy by reprogramming a herpes vi... Read More
The phase 1 study of CLBR001 + ABBV-461 is currently under way in the United States and is being developed in collaboration with AbbVie.
Trial NCT06878248 is an open-label, dose-escalation study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of the CLBR001 and ABBV-461 combination in patients with locally advanced or metastatic breast cancer. Participants receive a single infusion of CLBR001 following lymphodepletion, after which they are given cyclic doses of ABBV-461, with regular assessments to monitor safety and therapeutic response.
The two-component sCAR-T system includes CLBR001, an engineered autologous T cell product, and ABBV-461, an antibody-based biological drug. The switch is designed to bind to both the tumour-associated antigen and the CLBR001 cells, effectively activating the T cells only in the presence of the switch. This modular design gives physicians enhanced control over the therapy, including the ability to suspend the switch in the event of an adverse reaction.
Scripps Research is an independent, non-profit biomedical research institute consistently ranked among the most influential in the world for its innovation, according to the Nature Index. The organisation seeks to advance global health through transformative discoveries. Its drug discovery and development division, Calibr–Skaggs, works in close collaboration with multidisciplinary scientists to deliver new therapeutics efficiently.
ASCO'25: major advances in oncology therapies, research
The 2025 Annual Meeting of the American Society of Clinical Oncology (ASCO), this year held in Chicago, Illinois from May 30 to June 2, showcased a series of pivotal clinical trials and therapeutic... Read More
The Scripps Research Translational Institute applies genomics, digital medicine and data science to enhance individualised healthcare. Meanwhile, the Skaggs Graduate School trains the next generation of scientists, regularly ranked among the top 10 programmes in the United States for chemistry and biological sciences. Further information is available at www.scripps.edu.
The Calibr–Skaggs Institute for Innovative Medicines was founded on the principle that new medicines can be brought to patients more effectively by integrating leading biomedical research with advanced drug discovery. By leveraging the scientific foundation of Scripps Research, the institute has built a robust portfolio of investigational therapeutics based on proprietary technologies and is pioneering a new model for translating non-profit biomedical research into patient benefit. For further details, visit calibr.scripps.edu.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh