-
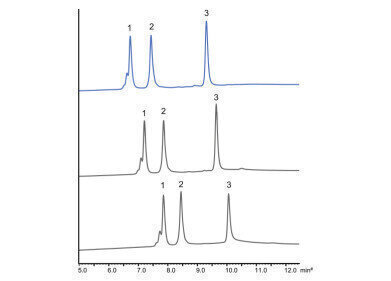 The new YMC Technical Note provides strategies to improve your HIC analysis, e.g. the separation of 3 commercial mAbs by varying the temperature.
The new YMC Technical Note provides strategies to improve your HIC analysis, e.g. the separation of 3 commercial mAbs by varying the temperature.
Chromatography
Technical Note: Strategies to Improve Your HIC Analysis
Sep 15 2022
Hydrophobic interaction chromatography (HIC) is a separation mode used for the analysis of biomolecules such as antibody-drug-conjugates (ADCs), monoclonal antibodies (mAbs) or proteins in general. Analytes are separated based on the differences in their surface hydrophobicity similar to reversed phase (RP) chromatography. However, a big plus is that analytes maintain their original structure and therefore functionality, making HIC a useful separation mode for intact protein analysis or protein purification.
HIC utilises reversible interactions that occur between the analyte and hydrophobic stationary phase ligands attached to the particle surface by applying an inverse salt gradient. A high salt concentration in the beginning of the gradient enhances the hydrophobic interactions between the target molecules and the stationary phase resulting in their retention. By decreasing the salt concentration, the strength of the interaction weakens and the analytes elute.
In this new Technical Note by YMC you will learn insights about:
- How to increase the retention by using elevated temperature and high initial salt concentrations
- The influence of salt concentration on sample precipitation
- Improving hydrophobic interactions by selecting the right pH
- How shallower gradients provide higher resolution
- The influence of organic modifiers on peak shape and retention
If you want to know more about the resolving power of non-porous HIC columns for intact proteins, check out this Technical Note.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh