-
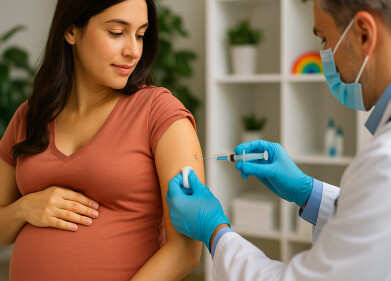
 L-R: Professor Gino Martini, PHTA CEO and Tim Henley, Lumir Clinic’s UK Director.
L-R: Professor Gino Martini, PHTA CEO and Tim Henley, Lumir Clinic’s UK Director.
Laboratory news
Strategic partnership to drive medicinal cannabis research in the UK
Mar 12 2025
Cannim, a leading Australian medicinal cannabis company, has announced its UK operations will be based at the Precision Health Technologies Accelerator (PHTA) in Birmingham. This strategic partnership aims to advance research and improve patient outcomes, particularly through the Lumir Clinic, Cannim’s dedicated research arm.
Founded in 2017, Cannim has focused on curating high-quality medicinal cannabis products from EU GMP-certified manufacturers. The collaboration with PHTA will enhance understanding of the endocannabinoid system, a crucial yet often overlooked part of the body that helps regulate balance. Phytocannabinoids, compounds found in cannabis, have shown potential in treating conditions like chronic pain, stress, and sleep disorders by interacting with this system.
As part of their partnership, Cannim and PHTA’s Lumir Clinic will launch observational clinical trials targeting veterans with PTSD. These trials will pave the way for future randomised controlled studies exploring the therapeutic benefits of medicinal cannabis. The research will also extend to other areas, including women’s health issues like dysmenorrhea and menopause, as well as age-related conditions such as Alzheimer’s disease.
The collaboration introduces a citizen science model, where patients will provide feedback on their experiences with medicinal cannabis. This valuable data will shape future research, ensuring treatments are developed with patient perspectives at the forefront.
“We’re excited to partner with PHTA and the University of Birmingham to explore the transformative potential of medicinal cannabis,” said Stuart Marsh, Chief Growth Officer of Cannim. “By combining our expertise with the University’s cutting-edge research, we aim to improve patient outcomes and deepen our understanding of how cannabis can help treat a variety of conditions.”
Though medicinal cannabis was legalised in the UK in 2018, it remains a prescription-only treatment, and there is still a knowledge gap within the medical community. Many doctors and patients are unaware that medicinal cannabis is available through prescriptions.
“We aim to provide evidence-based solutions and combat the stigma surrounding medicinal cannabis,” said Professor Anup Mathew, Medical Director of the Lumir Clinic. “Our goal is to ensure that both healthcare professionals and patients have access to the latest research and treatments.”
Professor Gino Martini, CEO of PHTA, added, “This partnership underscores the important role of the University of Birmingham and PHTA in advancing medical research. Together, Cannim and PHTA will help position Birmingham as a leading research hub for medical cannabis and cannabinoid studies.”
The Lumir Clinic is named after Professor Lumir Hanus, who discovered anandamide, a key molecule in the body’s endocannabinoid system involved in energy regulation.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh