-
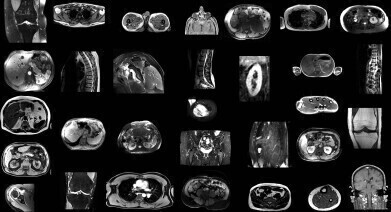 AI model automatically identifies structures from non-sequenced segments of MRI scans. Credit: University Hospital of Basel
AI model automatically identifies structures from non-sequenced segments of MRI scans. Credit: University Hospital of Basel
News
Artificial intelligence model automatically segments MRI scans
Mar 03 2025
Researchers in Switzerland have developed an artificial intelligence (AI) model that can automatically segment major anatomical structures in magnetic resonance imaging (MRI), independently of the sequence in which they were presented to the AI. The model outperformed other publicly available tools in the study.
MRI provides detailed images of the human body and has become an essential diagnostic tool for a variety of medical conditions, from neurological disorders to musculoskeletal injuries. For in-depth interpretation of MRI images, the organs, muscles and bones in the images are outlined or marked, a process which is known as segmenting.
“[Up to now] MRI images have been manually segmented, which is a time-consuming process that requires intensive effort by radiologists and is subject to inter-reader variability,” said Dr. Jakob Wasserthal, a research scientist withing the radiology department of University Hospital Basel in Basel, Switzerland.
“Automated systems can potentially reduce radiologist’s workload, minimise human error and provide more consistent and reproducible results,” he added.
Dr. Wasserthal and colleagues have built an open-source automated segmentation tool called the ‘TotalSegmentator MRI’ based on nnU-Net, a self-configuring framework that has set new standards in medical image segmentation. It adapts to any new dataset with minimal user intervention, automatically adjusting its architecture, preprocessing, and training strategies to optimize performance.
A similar model for computed tomography (CT) – TotalSegmentator CT – is being used by more than 300,000 users worldwide to process greater than 100,000 CT images daily.
In the retrospective study, the researchers trained TotalSegmentator MRI to provide sequence-independent segmentations of major anatomic structures using a randomly sampled dataset of 616 MRI and 527 CT exams.
The training set included segmentations of 80 anatomic structures typically used for measuring volume, characterising disease, surgical planning and opportunistic screening.
“Our innovation was creating a large data set,” Dr. Wasserthal said. “We used a lot more data and segmented many more organs, bones and muscles than has been previously done.
“Our model also works across different MRI scanners and image acquisition settings.”
To evaluate the model’s performance, Dice scores – which measure how similar two sets of data are – were calculated between predicted segmentations and radiologist reference standards for segmentations.
The model performed well across the 80 structures with a Dice score of 0.839 on an internal MRI test set. It also significantly outperformed two publicly available segmentation models (0.862 versus 0.838 and 0.560) and matched the performance of TotalSegmentator CT.
“To our knowledge, our model is the only one that can automatically segment the highest number of structures on MRIs of any sequence,” he said.
“It’s a tool that helps improve radiologists’ work, makes measurements more precise and enables other measurements to be done that would have taken too much time to do manually.”
In addition to research and AI product development, Dr. Wasserthal said the model could potentially be used clinically for treatment planning, monitoring disease progression, and opportunistic screening.
For further reading please visit: https://doi.org/10.1148/radiol.241613
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh