-
-
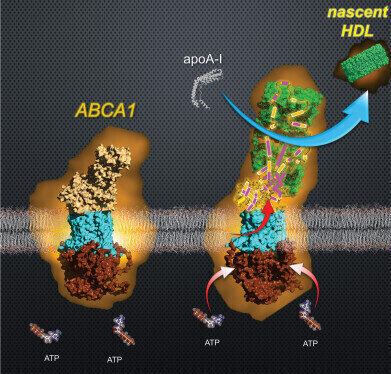 High-speed atomic force microscopy has enabled the visualization of ABCA1 in the process of generating nascent HDL. Credit: The authors
High-speed atomic force microscopy has enabled the visualization of ABCA1 in the process of generating nascent HDL. Credit: The authors
Microscopy & microtechniques
Researchers reveal mechanism behind formation of HDL ‘good cholesterol’
Oct 02 2025
Scientists in Japan have visualised how the transporter protein ABCA1 drives the formation of HDL widely known as ‘good cholesterol’. Using high-speed atomic force microscopy, the team showed how ABCA1 loads lipids onto apolipoprotein A-I, resolving a long-standing mystery in cholesterol biology and opening avenues for improved treatments of cardiovascular disease
High-density lipoprotein (HDL) often referred to as ‘good cholesterol’ plays a critical role in human health by removing excess cholesterol from tissues and transporting it to the liver. This process helps to prevent atherosclerosis, the accumulation of fatty plaques within arterial walls that can trigger life-threatening conditions such as heart attacks, strokes, aneurysms and blood clots. Yet despite their importance, the precise mechanisms by which HDL forms have long remained uncertain.
“It was historically believed that HDL pulled out excess cholesterol from cells through passive diffusion,” said Professor Kazumitsu Ueda of Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS).
“However, in 1999, a genetic analysis of Tangier disease, a condition characterised by low levels of blood HDL, revealed that the ATP-binding cassette protein A1 (ABCA1) – an ATP-dependent transporter – was essential for HDL production.
“That only deepened the mystery – how were HDLs being made, and what exactly were they doing?” he added.
Researchers at iCeMS have now employed a novel imaging methodology to answer this question. They demonstrated for the first time how ABCA1 generates HDL molecules, thereby offering new insight into one of the body’s most vital biochemical processes.
The team had initially proposed that ABCA1 would temporarily store about 500 cholesterol and phospholipid molecules within its extracellular domain, the part of the protein that extends beyond the cell. While the extracellular domain of ABCA1 is unusually large, earlier cryoelectron microscopy studies indicated that it formed a tunnel capable of holding fewer than ten lipid molecules at once. This discrepancy suggested that a more complex mechanism was occuring.
To test their ideas, Ueda’s group collaborated with colleagues at the Nano Life Science Institute (Nano-LSI) at Kanazawa University, Ishikawa, Japan led by Professor Noriyuki Kodera. The Kanazawa researchers had developed high-speed atomic force microscopy, a technology capable of visualising molecular events in real time at sub-second and nanometre-scale resolution.
“Few research groups in the world could perform this experiment,” said Ueda.
The observations revealed that HDL formation involved a dynamic sequence of events. ABCA1 used the energy released by the hydrolysis of adenosine triphosphate (ATP) to transfer lipids into its extracellular domain. The domain then underwent a dramatic expansion, creating a temporary structure that could store large amounts of lipids. These lipids were subsequently transferred en masse to apolipoprotein A-I (apoA-I), the main protein component of HDL particles. During this process, the extracellular domain of ABCA1 contracted sharply, losing about 30 per cent of its volume, and the resulting transfer produced nascent HDL particles.
“The physiological roles of HDL and cholesterol are often not fully understood.
“By clarifying the function and regulatory mechanisms of ABCA1, we hope to promote a more accurate understanding,” said Ueda.
The researchers believe that a clearer picture of how HDL forms, and of the precise function it performs, will enable future studies to examine the interplay between ‘good’ and ‘bad’ cholesterol with greater accuracy. Such insights could also contribute to therapeutic strategies for the prevention and treatment of cholesterol-related diseases.
Kodan highlighted the significance of the methodology itself, noting that the team’s use of high-speed atomic force microscopy allowed them to perform the technically challenging side-view imaging of membrane proteins.
“This novel methodology for efficient side-view imaging of human ABCA1 has the potential to be applied across a broad range of membrane protein systems, including the transport of lipids, drugs and metabolic products,” he said.
For further reading please visit: 10.1021/acs.nanolett.5c03116
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh
.jpg)
-(2).jpg)