-
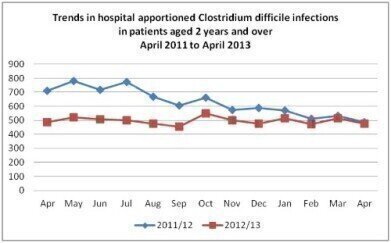 CDI continues to affect high numbers of patients in the UK and elsewhere
CDI continues to affect high numbers of patients in the UK and elsewhere
Microscopy & microtechniques
New CDI drug based upon human microbiome
Aug 01 2013
The US Food and Drug Administration (FDA) has approved a new Phase II clinical trial for a drug treatment of recurrent Clostridium difficile infection (CDI). Rebiotix Inc are set to begin the trial of RBX2660 following FDA approval. The drug is designed to help rebuild the intestinal microbiome in CDI sufferers.
The drug, RBX2660, contains live microbes that are designed to rebuild the intestinal microbiome so it is entirely healthy. If the Phase II study is successful, it could ultimately become the first drug based upon the microbiome in humans, approved by the FDA.
In the US alone, CDI affects 500,000 patients every year. It is one of the most common infections associated with health-care. Symptoms of the infection are most commonly presented as abdominal pain and profuse, watery diarrhoea.
Currently the only treatment available for patients to try and stop the disease from recurring is surgery, or antibiotics, neither of which are fully successful treatment options. Currently around 20 to 30 per cent of CDI sufferers experience a recurrence of the disease following initial treatment of commonly used first-line antibiotics. If a patient suffers from reinfection or has a number of relapses, the chances that CDI will recur are increased.
Rebiotix used a new investigational approach to the development of a recurrent CDI treatment. The company produced further research - to add to the growing body of information previously available - that indicates that an imbalance in intestinal flora may be responsible for allowing CDI to recur. The research suggests that the imbalance facilitates the processes of the disease, allowing it to return. The RBX2660 drug will correct this imbalance and therefore the natural functions of the intestines, stopping CDI from recurring.
The FDA awarded Rebiotix with 'fast track' status for RBX2660, which will allow for a quicker review of the data collected from its Phase II clinical trial. This could ultimately lead to the drug being made readily available for patients much quicker than usual clinical trial processes allow.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh
.jpg)
-(2).jpg)