-
 First author Samir Hossainy, a UChicago PME graduate student, works in the lab of Prof. Stuart Rowan. Credit: Photo by Jason Smith
First author Samir Hossainy, a UChicago PME graduate student, works in the lab of Prof. Stuart Rowan. Credit: Photo by Jason Smith -
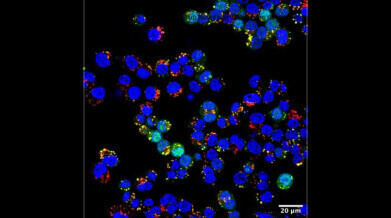 Researchers designed nanoparticles that can self-assemble at room temperature and deliver RNA (green) to living cells (nuclei shown in blue), offering a new pathway to vaccine and biologic drug design. Credit: Courtesy of Hossainy et al.
Researchers designed nanoparticles that can self-assemble at room temperature and deliver RNA (green) to living cells (nuclei shown in blue), offering a new pathway to vaccine and biologic drug design. Credit: Courtesy of Hossainy et al.
Research news
Nanoparticles that self-assemble at room temperature could transform vaccine delivery
Aug 18 2025
A team from University of Chicago Pritzker School of Molecular Engineering, Chicago, Illinois, United States has engineered polymer-based nanoparticles that self-assemble with a simple temperature shift. The discovery has the potential to broaden access to next-generation biologic medicines and vaccines.
“By simply warming a sample from fridge temperature to room temperature, we can reliably make nanoparticles that are ready to deliver a wide variety of biological drugs,” said co-senior author Dr. Stuart Rowan, the Barry L. MacLean Professor for Molecular Engineering Innovation and Enterprise at UChicago Pritzker School of Molecular Engineering and a staff scientist at Argonne National Laboratory.
Current lipid nanoparticle systems, which protected fragile drugs such as RNA and proteins in the development of SARS-CoV-2 mRNA vaccines, depend on alcohol-based solvents and sensitive steps during manufacture, rendering them poorly suited for protein delivery as well as being difficult to scale. The research team aimed to engineer a delivery system capable of working for both RNA and protein therapies. They sought a scalable method that did not require toxic solvents or complicated microfluidic systems.
Graduate student Samir Hossainy hypothesised that polymer-based nanoparticles could offer a more robust, customisable alternative. He established the necessary characteristics – the immune system only responded to particles of certain size, shape and charge – and then engineered polymers from first principles.
After testing and fine-tuning more than a dozen materials, Hossainy identified one that performed as required. At cold temperature the polymer and any desired protein remained dissolved in water; at room temperature the polymer self-assembled into uniformly sized polymersomes encapsulating the protein.
“Our particle size and morphology is dictated only by the chemistry of the polymers that [have been] designed from the bottom up.
“We do not have to worry about different particle sizes forming, which is a challenge with a lot of today’s nanoparticles,” Hossainy explained.
To assess the polymersomes, Hossainy collaborated with Rowan’s laboratory and with former UChicago PME Professor Jeffrey Hubbell, now of New York University. They demonstrated that the particles could encapsulate more than 75 per cent of protein and nearly 100 per cent of short interfering RNA cargo – far higher than most current systems – and that they could be freeze-dried and stored without refrigeration until needed.
In vaccination contexts, the team showed that polymersomes could carry a protein which, when administered to mice, induced durable antibody responses. Another experiment showed that proteins designed to suppress immune response in allergic asthma could be carried effectively. A further study indicated that injecting polymersomes into tumours could block cancer-related genes and suppress tumour growth in mice.
“This one formulation worked for everything we tried – proteins, RNA, immune activation, immune suppression and direct tumour targeting,” said Hossainy.
One of the most significant advantages of the polymersomes over lipid nanoparticles is the potential for low-tech, decentralised production. Hossainy envisaged shipping freeze-dried formulations worldwide; upon mixing in cold water and warming, the nanoparticles could be ready for administration.
“Being able to store these dry drastically improves the stability of the RNA or protein,” he noted.
The group is fine-tuning the particles to carry additional types of cargo, including messenger RNA like that which was used in some of the SARS-CoV-2 vaccines, although this is generally much larger than the siRNA used in the current study. They plan to collaborate on pre-clinical trials to apply the polymersomes to real-world vaccine or drug-delivery challenges.
For further reading please visit: 10.1038/s41551-025-01469-7
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















