-
 Researchers synthesize endo-functionalized oligophenylene cages using a novel template-assisted synthesis. Credit: Institute of Science Tokyo, Japan
Researchers synthesize endo-functionalized oligophenylene cages using a novel template-assisted synthesis. Credit: Institute of Science Tokyo, Japan
Research news
Template-guided synthesis enhances yield, functionality in oligophenylene molecular cages
Aug 06 2025
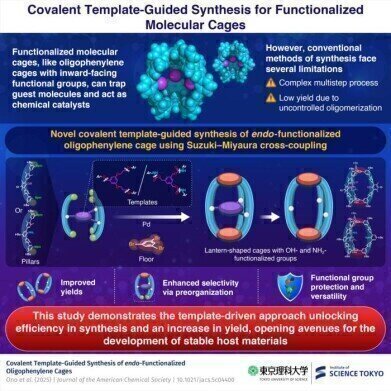
A research team in Japan has reported a novel and efficient method to synthesise endo-functionalised oligophenylene molecular cages using covalent templates. The strategy – which employed cooperative intermolecular coupling guided by a pre-organised molecular framework – achieved a yield of approximately 68% for hydroxyl-functionalised cages and 36% for amino-functionalised variants. These yields represent a considerable advance over conventional approaches, which typically achieve yields below 10%.
The study team was led by Associate Professor Kosuke Ono from the Department of Chemistry at the Institute of Science Tokyo. Their method involved a six-fold Suzuki–Miyaura cross-coupling reaction – a palladium-catalysed process – to form the covalent framework of the cages. The key innovation lies in the use of a covalent molecular template that directed the spatial orientation of reactants and promoted cooperative bond formation.
Molecular cages are hollow, cage-like molecular frameworks capable of encapsulating guest species. Among them, oligophenylene cages are valued for their chemical robustness, which arises from the stability of the phenylene backbone. However, their synthesis – particularly when seeking to introduce specific functional groups into the interior cavity – has remained challenging. Conventional synthetic routes often involve complex cyclisation reactions and suffer from extremely low yields, particularly in the case of endo-functionalised structures.
To address these limitations, Ono and his colleagues designed a strategy that began with the construction of a molecular cage precursor. This precursor comprised three quinquephenyl diboronic ester units arranged as vertical pillars, connected via a central covalent template. Subsequent coupling of this precursor to two tribromobenzene units formed the top and bottom of the lantern-shaped cage. This design enabled the controlled and simultaneous formation of six covalent bonds via the Suzuki–Miyaura reaction.
The use of a covalent template proved to be essential to the success of the methodology. It performed a dual function: not only did it bring the reacting moieties into close proximity, thereby enhancing the efficiency of bond formation, but it also acted as a protective scaffold, masking reactive functional groups during the coupling process. Following cage assembly, the template was chemically removed, revealing the desired internal functional groups – hydroxyl or amino – within the cavity of the cage.
“This represents the first efficient synthesis of oligophenylene cages with precisely modified internal spaces. The ability to construct such structures in a controlled and scalable manner opens the way to entirely new avenues in host–guest chemistry,” said Ono.
To confirm the structure and functionality of the synthesised cages, the team employed single-crystal X-ray diffraction. This technique validated the successful formation of the lantern-like architecture and revealed the precise orientation of the internal functional groups towards the cavity. These inward-facing groups enabled selective molecular encapsulation. For instance, hydroxyl-functionalised cages selectively entrapped L-tryptophan methyl ester, while amino-functionalised cages hosted diphenyl phosphate, thereby demonstrating the potential of the cages for selective molecular recognition.
The study also underscored the importance of cooperative reactivity in multibond-forming reactions. The researchers proposed that the formation of one bond facilitated the next, thereby lowering the overall energy barrier and increasing reaction efficiency. This cascade-like mechanism appears to be a critical feature of the template-guided synthesis.
According to the authors, the implications of this work extend beyond synthetic efficiency. The approach provides a rational design pathway for constructing functional molecular hosts with tailored internal environments. These cages may find application in supramolecular catalysis, molecular separation, chemical sensing, and controlled transport, among other areas.
“This is not merely a synthetic improvement but rather a conceptual advance that brings us closer to designing molecular architectures with predictable behaviour and high performance,” noted Ono.
The research continues to prompt further exploration into the scope and versatility of template-guided synthesis for the construction of complex, functional supramolecular assemblies. As such, it contributes significantly to the ongoing evolution of molecular design in organic and materials chemistry.
For further reading please visit: 10.1021/jacs.5c04400
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh