-
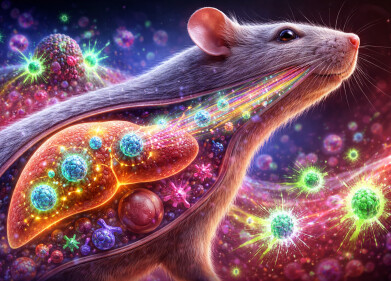
 Mechanism of pain relief by ADRIANA. Credit: KyotoU / Hagiwara lab
Mechanism of pain relief by ADRIANA. Credit: KyotoU / Hagiwara lab
Research news
Discovery of novel alternative to opioids offers hope for pain relief without risk
Aug 05 2025
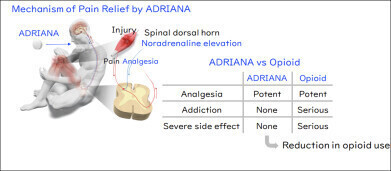
A novel oral analgesic developed at Kyoto University, Japan, has shown early promise as an alternative to opioids, with the potential to relieve pain without causing dependence or severe respiratory side effects. Researchers have described the drug – named ADRIANA – as the world’s first selective antagonist of the α2B-adrenoceptor. The compound is undergoing clinical development as part of an international collaboration, with a large-scale Phase II trial now planned in the United States.
The discovery has come at a time of acute concern over the misuse of synthetic opioids such as fentanyl – particularly in the US – where opioid overdoses caused more than 80,000 deaths in 2023. This escalating public health emergency has become widely known as the ‘opioid crisis’. In Japan, where opioids such as morphine are tightly regulated and available only by prescription from authorised physicians, there has been a strong impetus to develop alternative forms of pain relief.
“ADRIANA would offer a novel pain management option that does not rely on opioids, contributing significantly to the reduction of opioid use in clinical settings,” said corresponding author Masatoshi Hagiwara, a specially appointed professor at Kyoto University.
The Kyoto team was inspired by the body’s release of noradrenaline in life-threatening situations, which triggers the α2A-adrenoceptors and suppresses pain. While drugs that mimic noradrenaline can reduce pain, they carry a high risk of cardiovascular instability. Instead, the researchers hypothesised that to block α2B-adrenoceptors selectively could increase endogenous noradrenaline levels, thereby activating α2A-adrenoceptors and achieving pain suppression without cardiovascular side effects.
To explore this mechanism, the researchers used a novel method known as the transforming growth factor alpha (TGFα) shedding assay to identify selective α2B-adrenoceptor inhibitors. Through this screening process, they discovered a compound that selectively blocks α2B-adrenoceptors while preserving activity at other α2-adrenoceptor subtypes.
Preclinical studies in mouse models confirmed the compound’s safety and efficacy, which led to physician-led clinical trials at Kyoto University Hospital. Both the Phase I trial in healthy volunteers and the Phase II trial in patients with postoperative pain following lung cancer surgery yielded favourable results.
Based on these outcomes, Kyoto University and its spin-out company, BTB Therapeutics Inc., have begun preparations for a Phase II clinical trial in the US. The drug’s progression marks a significant milestone in Japan, where ADRIANA is the country’s first non-opioid analgesic to reach this stage of development.
“Pain has many forms, and we aim to evaluate ADRIANA’s effects across a range of pain types.
“Our goal is to make this treatment accessible to more patients living with chronic pain,” said Hagiwara.
The study, entitled: ‘Discovery and development of an oral analgesic targeting the α2B adrenoceptor’, was first published in Proceedings of the National Academy of Sciences of the United States of America on 4 August 2025.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh