-
-
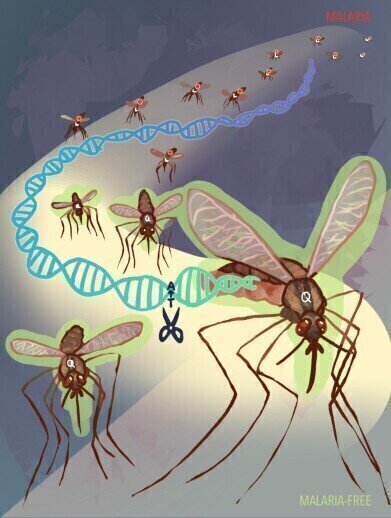 Mosquitoes that readily transmit malarial parasites carry the FREP1 amino acid known as L224 (red dots inside mosquitoes and marked with “L”). The newly developed system uses an allelic gene drive system (scissors) to convert mosquitoes into a population that now carries the malaria-suppressing Q224 allele. (highlighted in green and marked with “Q”). Credit: Audrey Yeun, Bier Lab, UC San Diego
Mosquitoes that readily transmit malarial parasites carry the FREP1 amino acid known as L224 (red dots inside mosquitoes and marked with “L”). The newly developed system uses an allelic gene drive system (scissors) to convert mosquitoes into a population that now carries the malaria-suppressing Q224 allele. (highlighted in green and marked with “Q”). Credit: Audrey Yeun, Bier Lab, UC San Diego
Research news
CRISPR-Cas9 gene edit in mosquitoes blocks transmission of malaria parasite
Jul 30 2025
Method swaps out a single amino acid which halts spread of disease
A collaborative research team has developed a novel genetic intervention in mosquitoes that appears to block transmission of the malaria parasite without harming the insects themselves. The novel approach – which only replaces a single amino acid in a key mosquito protein – has been designed to spread through insect populations and may offer a sustainable, genetically driven route to malaria control.
Malaria continues to present a major global health burden. In 2023, the disease reportedly infected 263 million people, causing almost 600,000 deaths, with four out of five fatalities occurring in children.
Efforts to curb transmission have stalled in recent years, largely due to mosquito populations developing resistance to insecticides and its parasite becoming resistance in its part to drugs. These setbacks have been compounded by disruption to eradication programmes compounded by the COVID-19 pandemic.
Researchers from the University of California San Diego, Johns Hopkins University in Baltimore, Maryland, the University of California at Berkeley and the University of São Paulo, Brazil have now developed a gene-editing system that uses CRISPR-Cas9 to introduce a single change to the FREP1 gene in the Anopheles stephensi mosquito, which is a major vector of malaria in Asia.
The edit swaps leucine at position 224 (L224) with a naturally occurring variant, glutamine (Q224), which appears to prevent the malarial parasites from being able to migrate to the mosquito’s salivary glands and thereby blocking transmission to a new host.
Professor George Dimopoulos of the Johns Hopkins Malaria Research Institute reported that the modified mosquitoes retained normal development and reproductive fitness, despite the disruption to its parasite’s life cycle. Laboratory tests showed the L224-to-Q224 switch successfully blocked two distinct species of Plasmodium – the parasites that cause malaria – from reaching the point of transmission.
“The beauty of this approach lies in leveraging a naturally occurring mosquito gene allele,” Dimopoulos said.
“With a single, precise tweak, we have turned it into a powerful shield that blocks multiple malaria parasite species and likely across diverse mosquito species and populations, paving the way for adaptable, real-world strategies to control this disease,” he added.
The gene-editing system has been engineered to function as an ‘allelic drive' – a mechanism that ensures the Q224 variant is preferentially inherited, allowing the resistance trait to spread throughout the mosquito population. This method builds on previous work from Professor Ethan Bier’s laboratory at UC San Diego, which has created gene drives that reverse insecticide resistance in crop pests.
“Replacing a single amino acid in mosquitoes with another naturally occurring variant that prevents them from being infected with malarial parasites – and spreading that beneficial trait throughout a mosquito population – is a game-changer,” said Bier, of the Department of Cell and Developmental Biology at UC San Diego.
“It is hard to believe that this one tiny change has such a dramatic effect,” concluded Bier.
Although the molecular mechanism behind the Q224 variant’s action remains incompletely understood, ongoing investigations aim to clarify how the amino acid disrupts parasite movement within the mosquito.
“This breakthrough is the result of seamless teamwork and innovation across institutions,” said Dimopoulos.
“Together, we have harnessed nature’s own genetic tools to turn mosquitoes into allies against malaria,” he added.
For further reading please visit: 10.1038/s41586-025-09283-6
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh