-
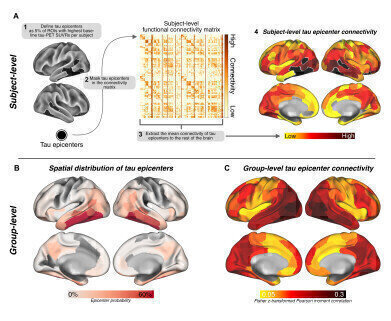 Caption: For each participant, 5% of brain regions with the highest baseline tau-PET SUVRs were defined as tau epicentres. (B) Epicentre masks were applied to (C) subject-specific connectivity matrices to (D) extract epicentre connectivity patterns. (E) Mapping of group-average epicentre probability and (F) epicentre connectivity. Credit: Roemer-Cassiano et al., Sci. Trans. Med. 17, eadp2564 (2025)
Caption: For each participant, 5% of brain regions with the highest baseline tau-PET SUVRs were defined as tau epicentres. (B) Epicentre masks were applied to (C) subject-specific connectivity matrices to (D) extract epicentre connectivity patterns. (E) Mapping of group-average epicentre probability and (F) epicentre connectivity. Credit: Roemer-Cassiano et al., Sci. Trans. Med. 17, eadp2564 (2025)
Research news
Detangling tau: Patient scans reveal how amyloid and tau proteins work in tandem in Alzheimer’s disease
Jan 22 2025
A comprehensive study of brain scans from more than 600 people has shed new light on the relationship between tau protein and amyloid-β, two central players in the progression of Alzheimer’s disease (AD). The findings provide insight into the disorder’s origins and could inform future strategies to target these proteins for therapeutic purposes. AD is intimately linked to accumulation in the brain tissue of amyloid-β and tau, leading to damaged neurons and subsequent cognitive decline. The relationship between tau protein and amyloid-β is still unclear and has slowed therapy development aimed at halting their accumulation.
One of the most popular models describing the onset of AD is the ‘amyloid cascade model’. This proposes that there is formation of amyloid-β plaques first which triggers the spread of tau proteins. In this study, Sebastian Roemer-Cassiano and colleagues examined brain imaging data from 140 patients and 69 healthy individuals enrolled in the AD Neuroimaging Initiative.
They analysed functional MRI data and PET scans on tau, glucose metabolism and amyloid-β spread gathered from the patients. The research discovered that brain regions affected by amyloid-β pathology exhibited higher neuronal activity and connectivity. Specifically, amyloid-β encouraged connections between ‘epicentres’ of tau in the temporal lobe, and other regions farther back in the brain (which previously have been shown to be more vulnerable to tau aggregates). The scientists replicated their findings in a second group of 345 people with preclinical AD and 55 healthy people.
“Together, our results should encourage others to further investigate neuronal activity and connectivity as key links between amyloid-β and tau to help specifically target the amyloid-β-tau axis in AD,” the team concluded.
A 2023 paper in Science Translational Medicine, by Walker et al, used proteomics and genomics on a cohort of middle-aged adults to examine the pathological changes involved in AD, who were followed-up longitudinally. This research identified pathway-specific plasma proteins that showed increased dementia risk up to 25 years later.
The pathway signature of these proteins was characterised by dysregulated immune signalling and proteostasis in the earliest preclinical stages and abnormal coagulation and complement signalling around 10 years before the onset of dementia. The study indicates that distinct biological mechanisms may be relevant in earlier and later preclinical stages of AD.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















