-
.jpg) [From left] Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim. Credit: KAIST
[From left] Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim. Credit: KAIST -
.jpg)
-
Research news
Neuroprotective fungal compound is synthesised in lab offering novel Alzheimer’s and Parkinson’s therapies
Aug 11 2025
Researchers at the Korea Advanced Institute of Science and Technology (KAIST) have reported the first total chemical synthesis of a rare class of natural products that have potent anti-neuroinflammatory properties and were previously only obtainable in trace amounts from fungi. The achievement could open nvoel pathways to develop therapeutics for neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
The team, led by Professor Sunkyu Han in the Department of Chemistry at KAIST, Daejeon, South Korea, has succeeded in synthesising herpotrichones A, B and C – compounds originally isolated from Herpotrichiasp. SF09, a fungus associated with its symbiotic host the ‘pill bug’.
These molecules possess a distinctive pentacyclic structure composed of four six-membered rings and one three-membered ring and have demonstrated both neuroprotective and anti-inflammatory properties in laboratory studies.
Pill bugs are also commonly known in British English as a ‘woodlouse’.
“Herpotrichone is the first rare natural product with pharmacological activity related to neurodegenerative diseases to be fully synthesised, and this work systematically presents the principle of biomimetic synthesis of complex natural products.
“It is expected to contribute to the development of novel natural product-based anti-neuroinflammatory therapeutics and biosynthesis research of this group of natural products,” said Professor Han.
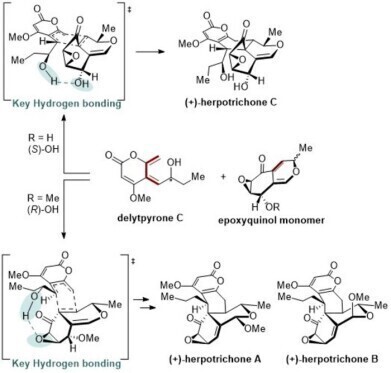
The team has devised a biomimetic synthetic route inspired by the biosynthetic origin of the herpotrichones. A key breakthrough was to apply the Diels–Alder reaction, a well-established method to construct six-membered carbon rings by joining two carbon-based partners in a concerted fashion.
To direct the stereoselectivity of this transformation and mimic the precision of enzyme-catalysed biosynthesis, the researchers introduced intramolecular hydrogen bonding. This design feature promoted chemo-, regio- and stereoselective formation of the desired products.
Hydrogen bonding played a critical role in stabilising the transition state during the Diels–Alder reaction, particularly by controlling the orientation of the C2′ hydroxyl group. This enabled selective synthesis of herpotrichone C, which was previously unknown at the time the KAIST group began work on herpotrichones A and B.
In 2024, the original Chinese research group that had reported the herpotrichones in 2019 identified herpotrichone C as a distinct natural product. The Korean team had independently synthesised the same structure as a major byproduct in earlier attempts to make A and B, thereby retrospectively confirming the structure of the newly reported molecule.
The synthetic route not only yielded the known natural products but also generated several structurally related compounds not previously observed in nature. These analogues may still be found to possess pharmacological properties of their own.
The study was published on 16 July in the Journal of the American Chemical Society, and lists Yoojin Lee, a student in KAIST’s integrated master’s and doctoral programme, as its first author.
For further reading please visit: 10.1021/jacs.5c05061
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















