-
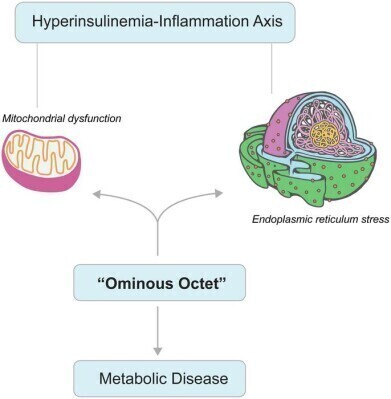 How mitochondrial dysfunction (MD) and endoplasmic reticulum (ER) stress act as central drivers of IR and metabolic disease progression. The reciprocal reinforcement between the dysfunction and hyperinsulinemia-inflammation cycle may ignite a self-propagating loop of metabolic disruption. Additionally, the diagram integrates MD and ER stress within the framework of the “Ominous Octet”, highlighting their pivotal role in metabolic dysregulation. Targeting both IR and its underlying cellular impairments is imperative for developing effective therapeutic interventions. Mitochondria and ER images were adopted from Pixabay. Credit: Swarup K. Chakrabarti
How mitochondrial dysfunction (MD) and endoplasmic reticulum (ER) stress act as central drivers of IR and metabolic disease progression. The reciprocal reinforcement between the dysfunction and hyperinsulinemia-inflammation cycle may ignite a self-propagating loop of metabolic disruption. Additionally, the diagram integrates MD and ER stress within the framework of the “Ominous Octet”, highlighting their pivotal role in metabolic dysregulation. Targeting both IR and its underlying cellular impairments is imperative for developing effective therapeutic interventions. Mitochondria and ER images were adopted from Pixabay. Credit: Swarup K. Chakrabarti
Research news
Immune system ageing identified as major factor in type 2 diabetes progression
Aug 19 2025
A recent review has synthesised evidence to demonstrate that immune ageing – marked by thymic involution, chronic low-grade inflammation, and immunosenescence (aging-related changes to the immune system) – acts as a significant accelerator of type 2 diabetes (T2DM) in older populations. The authors described how age-related immune decline interacts with cellular stress pathways to disrupt the so-called ‘ominous octet’ of eight interconnected organ dysfunctions that perpetuate hyperglycaemia, and consequently identifying potential targets for therapeutic intervention.
“Immune ageing is not merely a bystander but a catalytic force in the progression of T2DM,” the authors stated. They argued that integrating immunometabolic stress mechanisms into this established framework offers a route to preserve pancreatic β-cell function and immune resilience.
The review highlighted several mechanisms that link immune ageing to T2DM. Chronic low-grade inflammation, or ‘inflammaging’, results from the senescence-associated secretory phenotype, which releases pro-inflammatory cytokines such as interleukin-6 and tumour necrosis factor alpha. This process impairs insulin signalling, worsens insulin resistance and induces β-cell death through oxidative stress and endoplasmic reticulum dysfunction. Ageing also shifts macrophage activity from anti-inflammatory to pro-inflammatory phenotypes, further destabilising metabolic balance.
The authors detailed how compensatory hyperinsulinaemia, initially a protective response, becomes pathological. Elevated insulin levels activate stress kinases such as c-Jun N-terminal kinase and nuclear factor kappa B, which disrupt insulin receptor signalling and reduce glucose uptake. This fuels a self-reinforcing cycle in which inflammation and metabolic dysfunction accelerate β-cell exhaustion.
Organelle dysfunction emerged as a unifying pathway in disease progression. Mitochondrial impairment reduces adenosine triphosphate synthesis, increases reactive oxygen species, and disrupts calcium signalling, hampering insulin secretion and promoting excessive glucose production by the liver.
Endoplasmic reticulum stress leads to protein misfolding, activation of the unfolded protein response and inhibition of insulin receptor trafficking and glucose transporter type 4 translocation. Persistent stress triggers β-cell apoptosis via pro-inflammatory signalling pathways.
These immune and cellular stress mechanisms were shown to exacerbate all elements of the so-called ‘ominous octet’, including:
- β-cell failure through inflammasome activation
- hepatic overproduction of glucose
- increased lipolysis in inflamed adipose tissue
- reduced muscle glucose uptake
- upregulated renal glucose reabsorption
- diminished incretin secretion
- hypothalamic neurotransmitter dysregulation
- appetite control.
The authors proposed multi-targeted strategies to interrupt this immune-metabolic cycle. Suggested interventions include senolytic agents such as dasatinib and quercetin to remove senescent cells, specialised pro-resolving mediators to resolve inflammation, and glucagon-like peptide-1 receptor agonists to promote anti-inflammatory macrophage activity.
Approaches to protect organelle function – including mitophagy enhancement, unfolded protein response regulation and stabilisation of mitochondrial-endoplasmic reticulum contact sites – were also recommended. Biomarkers such as C-reactive protein, interleukin-6, and serine-phosphorylated insulin receptor substrate-1 were identified as possible tools to personalise treatment.
The review concluded that future research should account for differences in age and ethnicity and explore emerging fields such as gut microbiome-immune interactions, circadian rhythm disruption, and α- to β-cell transdifferentiation. The authors emphasised that a mechanistic understanding of immune ageing could enable the development of therapies that preserve metabolic control in ageing populations.
For further reading please visit: 10.14218/ERHM.2025.00018
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















