-
 LptM directly interacts with the folded LptDE complex and helps to stabilise its structure during outer membrane assembly. Credit: Ryoji Miyazaki, Nara Institute of Science and Technology, Japan.
LptM directly interacts with the folded LptDE complex and helps to stabilise its structure during outer membrane assembly. Credit: Ryoji Miyazaki, Nara Institute of Science and Technology, Japan.
Research news
Small protein found to stabilise gram-negative bacteria’s outer membrane structure, stability
Aug 08 2025
Researchers from Japan reveal that a remarkably small protein is essential for the maturation of a component of the lipopolysaccharide transport system
A Japanese research team has revealed the vital role of a small protein in the maturation and stability of a key component of the outer membrane in Gram-negative bacteria, offering potential insights into antibiotic development.
Gram-negative bacteria continue to present a global health threat due to their elevated resistance to antibiotics compared with gram-positive bacteria. This resistance arises in part from their robust outer membrane, which functions as a selective barrier to shield the cell from toxic substances. Far from being a passive structure, the outer membrane is essential to bacterial survival and virulence, prompting considerable scientific interest in how it is constructed and maintained.
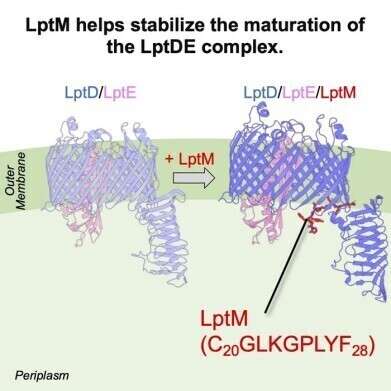
A central element in this process is the lipopolysaccharide transport system, which integrates lipopolysaccharide molecules into the outer membrane. While components such as the lipopolysaccharide transport DE (LptDE) complex have been recognised as essential for bacterial viability, the molecular mechanisms responsible for their maturation have remained incompletely understood.
A study team led by Assistant Professor Ryoji Miyazaki at the Nara Institute of Science and Technology has now identified a previously overlooked protein, named LptM, as critical for the proper assembly of the LptDE complex. The work, published online on 16 July 2025 and due to appear in Cell Reports on 26 August 2025 (Volume 44, Issue 8), was co-authored by Mai Kimoto, Dr Hidetaka Kohga, and Professor Tomoya Tsukazaki, also from the Nara Institute of Science and Technology.
The researchers combined biochemical techniques, mutational analysis, and cryo-electron microscopy to explore the function of LptM. They discovered that the protein acts during a later stage of LptD maturation, modifying intermediates that have already folded. A specific stretch of fewer than ten amino acid residues within LptM was found to be essential for its function. Structural data from high-resolution cryo-electron microscopy of the Escherichia coli LptDEM complex confirmed that LptM binds to a strategic interface in LptD, suggesting its role is to fine-tune the protein's conformation.
“Our study highlights the essential role of LptM, providing fundamental insights that may support antibiotic design, as the LptDE complex has been identified as a potential target for novel antibiotics,” said Dr Miyazaki.
The findings not only underscore the therapeutic potential of targeting the LptDE complex but also offer broader insights into bacterial cell biology. “Our findings suggest that small proteins, many of which have been previously overlooked, may play critical roles in the assembly and regulation of larger membrane protein complexes. This opens up a new perspective in basic biology, underscoring the functional relevance of small proteins,” Dr Miyazaki added.
The study has contributed to a growing body of evidence that small proteins, once thought to be biologically insignificant, may in fact be crucial actors in diverse cellular systems.
For further reading please visit: 10.1016/j.celrep.2025.116013
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















