-
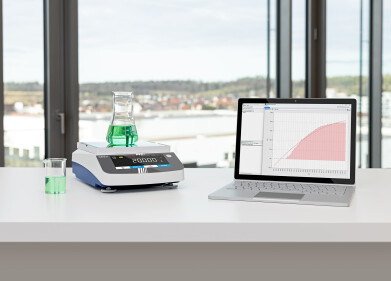
 FlowSyn continuous flow reactor system. (courtesy : Uniqsis)
FlowSyn continuous flow reactor system. (courtesy : Uniqsis) -
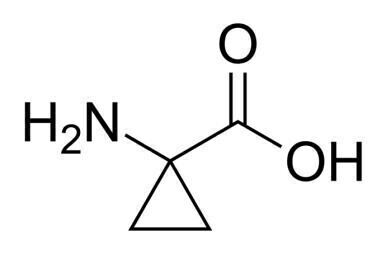 Example of a chiral Cyclopropane carboxylic acid.
Example of a chiral Cyclopropane carboxylic acid.
Laboratory products
Scalable flow method produces enantiomerically pure pharmaceutical intermediates
Mar 20 2024
Researchers at Lilly SA have pioneered a ground-breaking continuous flow method for the Wadsworth–Emmons cyclopropanation of alkyl-substituted chiral epoxides into chiral cyclopropane carboxylic acids, as reported by Uniqsis.
Utilising a custom setup comprising the FlowSyn continuous flow reactor and binary pump, the researchers successfully devised a novel flow process that overcame challenges associated with high temperature, pressure, and the volatile nature of the epoxide starting material.
The FlowSyn flow reactor demonstrated heightened efficiency by maintaining a higher concentration of low-boiling-point reactants in the liquid phase, due to its reduced headspace, resulting in enhanced reactivity compared to batch methods employing sealed vessels.
Furthermore, the researchers integrated their cyclopropane formation process with in-line workup and hydrolysis transformations, yielding enantiomerically pure cyclopropane carboxylic acids in impressive yields of up to 100 grams.
Uniqsis's FlowSyn, designed by a team of experienced flow chemists and engineers, stands as a fully integrated continuous flow reactor solution. Offering seamless reaction optimization and scale-up capabilities from milligrams to hundreds of grams, FlowSyn serves as a versatile tool for pharmaceutical intermediates production.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh