Laboratory products
Pharma and Nutra Labs: Addressing Sample Prep Bottlenecks in Elemental Analysis
Oct 01 2024
Accelerate the journey from sample to analysis with advanced workflow technologies.
INTRODUCTION
In 2018, the implementation of USP chapters 232, 233, and 2232 for heavy metal testing marked a significant milestone in elemental analysis for laboratories analyzing pharmaceutical and nutraceutical materials. Chapter 232 specifies the limits of elemental impurities for drug products, while 233 provides the analytical procedures for the measurements. Chapter 2232 defines the limits of elemental contaminants in dietary supplements. Over the years, positive outcomes have been observed, but the journey has also uncovered bottlenecks in sample preparation that hinder labs from reaching their full potential in terms of efficiency, cost-effectiveness, productivity, and safety.
However, there are ways to address the various pain points in the workflow. For example, solutions are available for digestion challenges, including running mixed samples, avoiding sample reprocessing, and ensuring interference-free solutions for analysis. Evaluating the Total Sample Preparation Workflow offers the ability to tackle obstacles along every step of the path from sample to analysis. Each laboratory operation can be optimized through automation, which accelerates turnaround time, achieves higher quality results, and improves operator safety. Taking a practical, strategic approach to every procedure helps prevent workflow disruptions that negatively affect laboratory performance.
PAIN POINTS IN THE ELEMENTAL ANALYSIS WORKFLOW
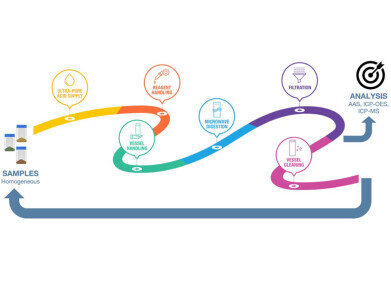
The workflow in Figure 1 depicts the many steps involved in sample preparation for elemental analysis. The ultimate goal is to obtain high quality results in a timely and safe manner. Because every step of the workflow affects the quality of the results as well as lab throughput, turnaround time (TAT), costs, and safety, each of them should be carefully considered to attain the best outcome.
Within this workflow, there are multiple pain points that are common to both pharmaceutical and nutraceutical laboratories that must be addressed. It is not unusual for labs to receive varied sample types that require different methods in a typical rotor-based digestion system. Attempts to run disparate samples together often results in incomplete digestion. Additionally, some pharmaceutical samples are prone to exothermic reactions that must be controlled. Sample processing times can be very important for meeting requirements for turnaround times as well. Acid purity and handling are also critical; ultra-pure acid must be utilized according to USP 233 procedures, and handling of sample preparation components minimized to prevent contamination and ensure operator safety. The workflow requires manual labor, which calls for hiring laboratory staff and providing training. Cleaning the various pieces of labware is also time consuming but vital.
In the fourth step of the workflow, incomplete digestion is a serious problem that can have a variety of causes. For example, the final digestion temperature may not be hot enough. It is important to remember that the temperature drives the quality of digestion and pressure is simply a by-product of it. In other cases, the digestion time may be too short to achieve complete digestion. Employing incorrect chemistry or volumes of acid will also lead to incomplete digestion. Sufficient acid volume is necessary to break down all the chemical bonds, and utilizing the wrong acid is akin to using the wrong key to a lock. Typically, in pharma/nutra labs, nitric acid is used as an oxidizing acid, hydrogen peroxide is often added for rotor-based systems, and hydrochloric acid is used to stabilize mercury. Signs of incomplete digestion include yellow coloring (due to residual carbon content from undigested carbon-carbon bonds), cloudiness, and unexplained sediment. Selecting the appropriate sample digestion technology and conditions is therefore critical to meeting a laboratory’s data quality and turnaround time objectives.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh