Clinical, medical and diagnostics
As a reliable manufacturer of the original Dialysate Meter™, Myron L® Company has served haemodialysis professionals for over 50 years. This powerful, portable Digital Dialysate Meter™ features extreme accuracy in widely varying ranges, easy one-touch measurements, and keypad calibration.
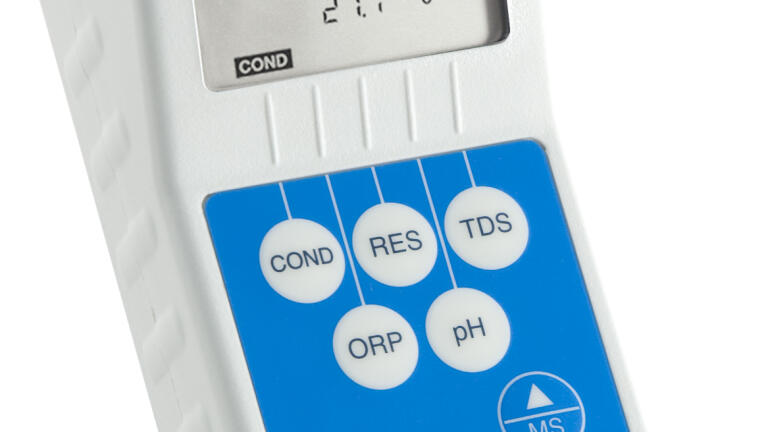
Model D-6 is designed to test both acetate and bicarbonate dialysate quality. Measure Conductivity, pH, and Temperature to ensure proper mixing during dialysate preparation and as a final check of dialysate quality before haemodialysis treatment. There are no strips or reagents that expire or subjective interpretation of results. Internal sensors require the user to place the sample in the instrument, preventing contamination of dialysate by the measurement device. Digital Dialysate Meters do not need sterilisation, and you never have to return an instrument to the manufacturer for calibration.
The D-6 also measures parameters critical in water quality monitoring and control at every stage of haemodialysis water treatment. Test Conductivity, Resistivity, TDS, pH, ORP, Free Chlorine Equivalent (FCETM) and Temperature of feed and product water in unit processes. Use measurements as independent checks against automatic systems of control and to check residual levels of sanitisers in water treatment and haemodialysis systems after system maintenance.
The D-6 is low maintenance with an easy-to-use interface. Technicians only need to learn, use and train on one instrument for all water testing. Factory programmed solution models for dialysate and haemodialysis water quality testing increase accuracy. Conductivity/TDS/Resistivity measurement accuracy is ±1% of reading vs. full scale. pH measurement accuracy is ±.01 pH units. The LCD display is a full, four-digit display with superior resolution.
The D-6 can store 100 readings with date & time stamp. Bluetooth® communication is available with the bluDock™ option for easy record-keeping. And live technical support with little to no wait time is included for the life of the product.
The D-6 DIGITAL DIALYSATE METER has received FDA 510(k) acceptance for use as a medical device for testing dialysate. To view the Myron L® Company DIGITAL DIALYSATE METER 510(k) follow this link.
More information online