-
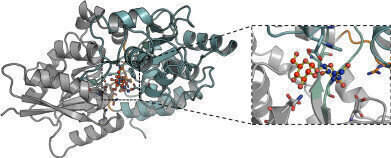 Molecular-level detail of the interaction of trehalose bound to the mycobacterial LpqY transporter. (Credit: University of Warwick)
Molecular-level detail of the interaction of trehalose bound to the mycobacterial LpqY transporter. (Credit: University of Warwick)
News
Targeting Uptake Source could fight TB
Apr 27 2021
Tuberculosis (TB), caused by the bacterial pathogen Mycobacterium tuberculosis (Mtb) is the leading cause of death from a single infectious agent world-wide claiming over 1.5 million lives each year. With an increase in resistance to current antibiotics means that novel drugs to kill Mtb are urgently needed. Researchers from the University of Warwick have successfully discovered how Mycobacterium tuberculosis uses an essential sugar to survive in the human body for long time periods; this provides a platform to design new and improved TB drugs and diagnostic agents.
Trehalose is one source of energy found in the pathogen’s own cell wall and it appears that Mtb has evolved a strategy to recycle and reuse this sugar as a valuable food source.
The transport protein LpqY, which is responsible for the uptake of trehalose, is essential for Mtb to establish infection. If the LpqY protein is deleted and no longer able to function then Mtb can no longer supply itself with trehalose and becomes less pathogenic.
The researchers from Warwick’s School of Life Sciences used X-ray crystallography to determine the 3-dimensional structure of LpqY and analysed how this important transport protein is able to bind and recognise trehalose. They then went on to use a number of experimental techniques which showed that while LpqY is highly specific for trehalose, it is also able to recognise sugars that are similar to trehalose with small modifications and map key recognition features.
Dr Elizabeth Fullam, who is a Sir Henry Dale Fellow from the School of Life Sciences at the University of Warwick commented:
“It is vital that we find new innovative strategies to combat TB. The LpqY trehalose transporter is a potential drug target because when it is not functioning it results in Mtb becoming less virulent. Now that we understand exactly how trehalose is recognised we will be able to design specific molecules that will enable us to kill TB. Alternatively, another possibility is that we can use the LpqY transporter to our advantage and find ways to deliver compounds for TB diagnosis.”
The researchers acknowledge the funding for this work which is supported by a Sir Henry Dale fellowship awarded to Dr Elizabeth Fullam, jointly funded by the Wellcome Trust and The Royal Society.
The paper, ‘Structural basis of trehalose recognition by the mycobacterial LpqY-SugABC transporter’, is published in the Journal of Biological Chemistry.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















