News
Alzheimer’s blood test trial in the UK could transform diagnosis and speed access to treatment
Sep 10 2025
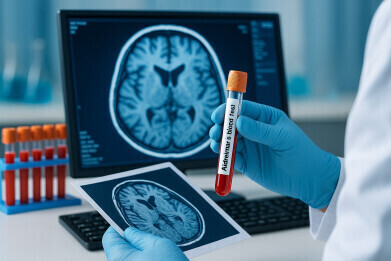
A UK-wide clinical trial has begun to evaluate a blood test that can detect Alzheimer’s disease with more than 90% accuracy. University College London scientists believe the test, which measures the biomarker p-tau217, could replace invasive brain scans and lumbar punctures, help clinicians deliver faster diagnoses, and prepare patients for novel treatments now in late-stage development
More than 1,000 people in the United Kingdom with suspected dementia are to be offered a blood test for Alzheimer’s disease in a trial that researchers believe could transform the accuracy of diagnosis.
The study, led by scientists at University College London, will assess how the test performs across the UK’s National Health Service. Patients are to be recruited at 20 memory clinics. Researchers have said the test could raise diagnostic accuracy from 70% to greater than 90% and offer benefits to both patients and clinicians. Recruitment for the Alzheimer’s Disease Diagnosis and Plasma p-tau217 (ADAPT) trial has begun.
Alzheimer’s disease, the most common form of dementia, is caused by the build-up of two rogue proteins – amyloid and tau – which may accumulate in the brain for up to 20 years before symptoms appear. The blood test measures a biomarker called p-tau217, which reflects the presence of both proteins, and currently costs around £100 to perform.
Until now, confirmation of Alzheimer’s has required specialist positron emission tomography (PET) brain scans or lumbar punctures to analyse cerebrospinal fluid. These ‘gold standard’ tests are not part of routine diagnosis and fewer than 2% of patients receive them.
“Our recent Lived Experience Survey revealed that only a third of people with dementia felt their experience of the diagnosis process was positive, while many reported being afraid of receiving a diagnosis.
“As a result, too often, dementia is diagnosed late, limiting access to support, treatment and opportunities to plan ahead,” said Professor Fiona Carragher, chief policy and research officer at the Alzheimer’s Society.
The trial has been launched as part of the Blood Biomarker Challenge and is supported by Alzheimer’s Research UK and the Alzheimer’s Society, with funding from the People’s Postcode Lottery.
Jonathan Schott, professor of neurology at University College London and chief medical officer at Alzheimer’s Research UK, said he was “thrilled” to welcome participants onto the ADAPT trial.
“It’s a critical part of the Blood Biomarker Challenge, which we hope will take us a step forward in revolutionising the way we diagnose dementia,” he said.
Half the participants will receive their results within three months, while the others will be told after 12 months. Researchers will examine whether earlier feedback accelerates diagnosis, guides clinical decision-making, and affects how patients and doctors respond to results. The trial will also assess the effect of early testing on quality of life.
If successful, the test could become a standard tool in Alzheimer’s diagnosis. Researchers have said this will be crucial as several novel drugs for early-stage disease approach regulatory approval.
The research team expects to publish its results within three years.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh