-
-
 [From left] Dr Giuseppe Battaglia, Catalan Institution for Research and Advanced Studies research professor at the Institute for Bioengineering of Catalonia (IBEC), Principal Investigator of the Molecular Bionics Group and leader of the study, and Dr. Lorena Ruiz Pérez, senior researcher in the Molecular Bionics Group at IBEC, associate professor at the University of Barcelona and co-author of the study. Credit: Institute for Bioengineering of Catalonia
[From left] Dr Giuseppe Battaglia, Catalan Institution for Research and Advanced Studies research professor at the Institute for Bioengineering of Catalonia (IBEC), Principal Investigator of the Molecular Bionics Group and leader of the study, and Dr. Lorena Ruiz Pérez, senior researcher in the Molecular Bionics Group at IBEC, associate professor at the University of Barcelona and co-author of the study. Credit: Institute for Bioengineering of Catalonia -
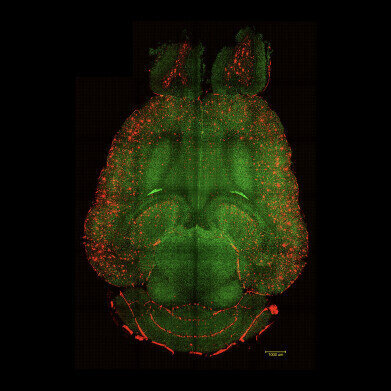 Light sheet fluorescence microscope images of mouse brain 12h after NOT being treated with nanoparticles. The brains were analysed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia
Light sheet fluorescence microscope images of mouse brain 12h after NOT being treated with nanoparticles. The brains were analysed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia -
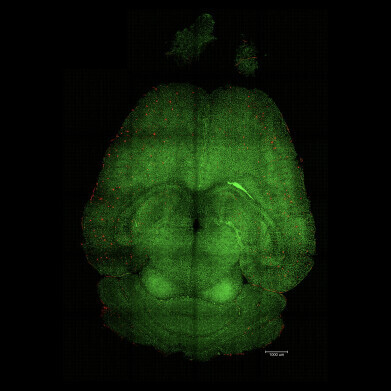 Light sheet fluorescence microscope image of mouse brain 12h after being treated with nanoparticles. The brains were analysed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia
Light sheet fluorescence microscope image of mouse brain 12h after being treated with nanoparticles. The brains were analysed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia
Research news
Scientists report striking reversal of Alzheimer’s using vascular repair technology in mice
Oct 13 2025
Researchers from the Institute for Bioengineering of Catalonia and West China Hospital Sichuan University have reported a striking reversal of Alzheimer’s pathology in mice by restoring the blood–brain barrier rather than targeting neurons. Their supramolecular drug nanoparticles achieved rapid amyloid-β clearance, re-establishing vascular health and cognitive function in preclinical models
An international team co-led by the Institute for Bioengineering of Catalonia (IBEC), in Barcelona and West China Hospital Sichuan University (WCHSU) in Chengdu, China has reported a major advance in the research of Alzheimer’s disease. The researchers have demonstrated that a nanotechnology strategy can reverse Alzheimer’s pathology in mice by restoring functionality in the blood–brain barrier (BBB) rather than acting directly on neurons.
Nanomedicine hitherto has typically used nanoparticles as couriers for therapeutic molecules. However, in this study, the team deployed nanoparticles that were bioactive in their own right – so-called ‘supramolecular drugs’. By repairing the BBB – the vascular gatekeeper that regulates the brain’s chemical environment – the investigators brought about a reversal of the pathological features of Alzheimer’s disease in mouse models.
The brain is the most energy-demanding organs, alone consuming around 20 per cent of an adult’s total energy budget and can be up to 60 per cent in children. Its energy supply depends on an extensive vascular network in which each neuron is nourished by at least one capillary. The human brain contains approximately one billion capillaries, underscoring the critical role vascular health plays in both normal brain function and disease states like Alzheimer’s where vascular dysfunction is increasingly implicated.
The BBB is a selective cellular and physiological barrier separating brain tissue from the circulating blood. It protects the brain from pathogens and toxins while regulating the transport of essential molecules. In Alzheimer’s disease, accumulation of so-called ‘waste proteins’ – chiefly amyloid-β (Aβ) – impairs neuronal function. The research team showed that by targeting a specific mechanism, they could (re-)allow Aβ trapped in the brain to cross the BBB and enter the bloodstream for elimination.
The investigators used transgenic mouse models engineered to overproduce Aβ and to develop cognitive decline mimicking Alzheimer’s pathology. The protocol administered just three doses of the supramolecular drugs while the team monitored disease progression over time.
“[At just] one hour after the injection we observed a reduction of 50–60 per cent in Aβ amount inside the brain,” said Junyang Chen, first co-author, researcher at WCHSU and also a PhD doctoral candidate at University College London, UK.
The most striking observations emerged in functional and behavioural assessments. The researchers conducted experiments across disease stages to assess memory and cognition over periods of months. In one trial, they treated a 12-month-old mouse – analogous to a 60-year-old human – and tracked its behaviour for six further months. At 18 months of age – so roughly equivalent to 90 years old – the treated mouse had regained behaviour typical of a healthy subject.
“The long-term effect comes from restoring the brain’s vasculature. Once the vasculature is able to function again, it starts clearing Aβ and other harmful molecules, allowing the whole system to recover its balance,” said Dr. Giuseppe Battaglia, ICREA Research Professor at IBEC, principal investigator of the Molecular Bionics group.
“What’s remarkable is that our nanoparticles act as a drug and seem to activate a feedback mechanism that brings this clearance pathway back to normal levels,” he added.
In Alzheimer’s disease, one major issue is failure of the brain’s natural clearance system for toxic species such as amyloid-β. Under normal conditions, the protein low-density lipoprotein receptor-related protein 1 (LRP1) functions as a molecular gatekeeper: it recognises Aβ, binds to it via ligands, and ferries it across the BBB into the bloodstream for disposal. But the system is delicate: if LRP1 binds too tightly to Aβ, the transport route may clog up or the receptor may degrade; and if the binding is too weak transport can fail. In both cases, Aβ accumulates in brain tissue with negative outcomes.
The supramolecular drugs developed in this work act like a molecular switch to reset the system. By mimicking LRP1’s ligands, they bind to Aβ, can cross the BBB, and initiate a clearance cascade that restores the vascular pathway’s natural waste-clearing function. The result is both the removal of toxic species and rebalancing of vascular regulation.
These nanoparticles were engineered via a bottom-up molecular design, allowing precise control of their size and the number of surface ligands. This multivalent architecture enables highly specific interaction with cellular receptors and facilitates modulation of receptor trafficking at the cell membrane level – a novel method by which to influence receptor function. The precision built into these supramolecular drugs underpins both effective Aβ clearance and restoration of vascular balance in the brain.
This therapeutic paradigm opens a promising pathway for designing interventions that directly address vascular dysfunction in Alzheimer’s disease.
“Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance, restoring healthy function in the blood–brain barrier and leading to a striking reversal of Alzheimer’s pathology,” said Dr. Lorena Ruiz Pérez, senior researcher in the Molecular Bionics group at IBEC and an associate professor at the University of Barcelona.
The study represented a collaboration between IBEC, WCHSU, West China Xiamen Hospital of Sichuan University, University College London, the Xiamen Key Laboratory of Psychoradiology and Neuromodulation, University of Barcelona, the Chinese Academy of Medical Sciences and the Catalan Institution for Research and Advanced Studies.
For further reading please visit: 10.1038/s41392-025-02426-1
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh