-
-
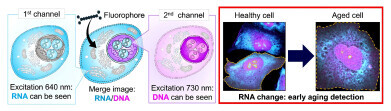 Using two types of harmless red-to-near-infrared light, both DNA and RNA can be distinguished simultaneously inside a cell (left), and cell aging progression can be observed (right). Credit: Linawati Sutrisno, National Institute for Materials Science; Katsuhiko Ariga, National Institute for Materials Science
Using two types of harmless red-to-near-infrared light, both DNA and RNA can be distinguished simultaneously inside a cell (left), and cell aging progression can be observed (right). Credit: Linawati Sutrisno, National Institute for Materials Science; Katsuhiko Ariga, National Institute for Materials Science
Research news
Infrared DNA and RNA imaging method maps all stages of living cells' death
Nov 27 2025
Researchers from the National Institute for Materials Science in Japan and international partners have reported a novel infrared DNA and RNA imaging technique that tracks all stages of cell damage and cell death in living cells, which could support ultra-early diagnosis, drug screening and precision medicine
Researchers at the National Institute for Materials Science (NIMS) in Japan, together with colleagues at Nagoya University, Gifu University, all in Japan, and the University of Adelaide in South Australia, have developed a method to image deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) simultaneously inside living cells by use of harmless infrared to near-infrared light. The work enables high-precision detection of all stages of cell death and has opened a route to ultra-early detection of cellular ageing and damage for disease prevention.
Early identification of cellular damage that leads to ageing or death is essential to develop therapeutic strategies for many common and serious diseases, from neurodegeneration to cancers. To achieve this, researchers must observe how cells respond to stress and treatment across the entire cellular life cycle. Live-cell imaging has become a central tool for this task.
Current imaging methods, however, often rely on ultraviolet or visible light that can be harmful, alter cell behaviour or damage their genetic material. Many techniques also struggle to distinguish early and late injury states or to resolve multiple forms of cell damage in the same specimen. These limitations can delay diagnosis, obscure the true fate of cells after treatment and lead to misinterpretation of therapeutic effects.
To address these challenges, the NIMS-led team designed a universal, highly sensitive imaging platform that relies on gentle infrared to near-infrared excitation. The system uses two types of fluorescent dye probe – based on N-heteroacene molecules – which bind in different ways to DNA and RNA. By illumination with two harmless excitation wavelengths, the method can visualise both nucleic acids within the same live cell without toxic labelling steps or destructive sample preparation.
In experiments on living cells, the researchers showed that DNA-selective imaging could reveal sustained DNA damage, such as that which arises after exposure to strong chemicals or physical injury. However, they also found that RNA-selective imaging provided greater sensitivity to early cell stress and ageing. Subtle changes in RNA distribution and signal intensity appeared before irreversible DNA damage became obvious. According to the authors, this means that RNA signals can act as an early-warning readout for cell fate, while DNA signals report more advanced damage.
By combining the two readouts, the dual DNA/RNA imaging approach enabled precise detection of four stages of cell death considered by the study – apoptosis (programmed cell death), necrosis (accidental cell death), necroptosis (a programmed form of necrosis which uses a defined molecular pathway) and cellular senescence (cells enter a state of permanent growth arrest, no longer dividing, but remain metabolically active) – and resolved how individual cells progressed from healthy states through intermediate damage to terminal death.
Crucially, the system tracked these transitions at the single-cell level allowing the team to monitor the heterogeneous responses that bulk assays can often miss. The method therefore surpassed the limits of many existing imaging systems that cannot follow fine-grained state transitions in individual cells in real time.
The authors suggested that this infrared-based DNA/RNA imaging platform could possibly allow for ultra-early detection of cellular damage and ageing in live samples, without the potential for damage that can accompany UV-based techniques. Because the excitation light lies in a biologically friendly window and the dyes operate at low intensity, the method suits long-term observation and non-invasive live-cell diagnostics.
The method also appears highly compatible with automated microscopes and microplate formats which makes it attractive for high-throughput drug screening workflows that require simultaneous monitoring of cell viability, stress and death pathways across large cell populations.
The team has indicated that it now plans to extend the method from cultured cells to intact tissues and whole organisms. The goal is to establish practical techniques to support early disease detection, monitoring of cellular stress in vivo and refinement of precision medical strategies that tailor interventions to subtle cellular changes.
In the longer term, the researchers envisage diagnostic technologies that could identify a cellular ‘pre-disease’ state – a stage in which a person has started to drift away from health – solely by observation of cell-level DNA and RNA signals before clinical symptoms appear.
For further reading please visit: 10.1126/sciadv.adz6633
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh