-
-
 Researchers propose a four-tiered framework to investigate the sleep–brain–gut axis. First, human studies reveal altered gut microbiota in people with sleep disorders. Second, machine learning integrates genomic and clinical data to identify biomarkers for personalised treatments. Third, faecal microbiota transplantation establishes causal links between microbial changes and sleep outcomes. Finally, randomised controlled trials and crossover studies assess microbiome-based therapies using specific microorganisms and metabolites to improve sleep quality and restore physiological balance. Credit: Lin Lu
Researchers propose a four-tiered framework to investigate the sleep–brain–gut axis. First, human studies reveal altered gut microbiota in people with sleep disorders. Second, machine learning integrates genomic and clinical data to identify biomarkers for personalised treatments. Third, faecal microbiota transplantation establishes causal links between microbial changes and sleep outcomes. Finally, randomised controlled trials and crossover studies assess microbiome-based therapies using specific microorganisms and metabolites to improve sleep quality and restore physiological balance. Credit: Lin Lu -
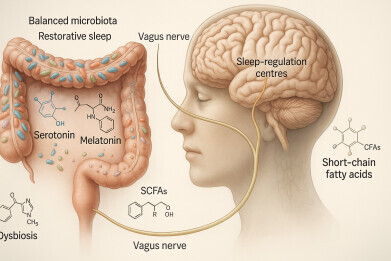
 The microbiota–gut–brain axis regulates sleep and wakefulness through immune, neural and metabolic–endocrine pathways. Gut microbes produce metabolites such as butyrate, GABA and melatonin that promote sleep, while serotonin, orexin and histamine support wakefulness. Signals travel via the vagus nerve, bloodstream and hypothalamic–pituitary–adrenal axis, linking gut activity to sleep-related brain regions. This bidirectional system ensures dynamic balance, with changes in gut composition or stress hormones influencing sleep regulation and overall physiological stability. Credit: Lin Lu
The microbiota–gut–brain axis regulates sleep and wakefulness through immune, neural and metabolic–endocrine pathways. Gut microbes produce metabolites such as butyrate, GABA and melatonin that promote sleep, while serotonin, orexin and histamine support wakefulness. Signals travel via the vagus nerve, bloodstream and hypothalamic–pituitary–adrenal axis, linking gut activity to sleep-related brain regions. This bidirectional system ensures dynamic balance, with changes in gut composition or stress hormones influencing sleep regulation and overall physiological stability. Credit: Lin Lu
Research news
Gut microbiota emerges as key regulator of sleep through microbiota–gut–brain axis
Nov 10 2025
A comprehensive review has revealed that gut bacteria play a pivotal role in regulating sleep, linking microbial imbalance to insomnia, obstructive sleep apnoea and other disorders. The study identifies therapeutic prospects for microbiota-targeted interventions such as probiotics, prebiotics and faecal-microbiota transplantation
A comprehensive review has revealed how gut microbiota influences sleep regulation, establishing the microbiota–gut–brain axis as central to understanding and potentially treating sleep disorders. The study, led by Professor Lin Lu of Peking University Sixth Hospital in Haidian, Beijing, China – with international collaborators across China and the United States – synthesises current knowledge on how the trillions of bacteria within the digestive tract affect sleep–wake cycles through neurological, metabolic and immune pathways.
Sleep disorders affect millions worldwide, with chronic insomnia, obstructive sleep apnoea and circadian rhythm disturbances undermining physical and mental health. While much progress has been made in mapping central nervous system mechanisms of sleep, this review highlights that peripheral organs, particularly the gut, play an equally critical role.
The human intestine hosts a diverse microbial ecosystem that communicates bidirectionally with the brain. Pathways include direct neural connections via the vagus nerve, immune signalling and the generation of bioactive metabolites capable of crossing the blood–brain barrier.
“The gut microbiota is increasingly recognised as a key player in neurological and psychiatric health.
“Our review demonstrates that disruptions in gut microbiota composition are closely linked to sleep disturbances across multiple disorders,” Professor Lu explained.
Clinical and preclinical studies indicate consistent microbial imbalance – known as dysbiosis – in individuals with disrupted sleep. Patients with chronic insomnia display reduced microbial diversity and altered bacterial families compared with healthy controls. Similar patterns appear in obstructive sleep apnoea, where lower abundances of beneficial bacteria correlate with more severe symptoms.
Recent work has shifted from simple correlations to molecular investigations of how microbial products affect neurophysiology. The review identifies several key biological mechanisms, including short-chain fatty acids such as butyrate, which protect against sleep disruption by reducing inflammation and strengthening intestinal barriers. Clinical trials have shown sodium butyrate to improve sleep quality in ulcerative-colitis patients, while animal studies demonstrate that butyrate mitigates inflammation and memory impairment caused by sleep deprivation.
Bile acids form another crucial link between gut bacteria and sleep. Chronic insomnia correlates with increased concentrations of primary bile acids and reductions in secondary bile acids; a pattern associated with decreased Ruminococcaceae species and higher cardiometabolic risk. The microbiota–bile-acid relationship therefore appears integral to the broader physiological effects of poor sleep.
Gut microbes also influence neurotransmitter production. Certain Lactobacillus and Bifidobacterium strains encode enzymes that synthesise gamma-aminobutyric acid (GABA), a neurotransmitter that promotes sleep. Electroencephalography studies show that GABA intake modulates brain activity, supporting the notion that gut-derived GABA can affect sleep architecture.
Similarly, more than ninety per cent of serotonin is produced in the gut, with microbial metabolism of tryptophan shaping serotonin and melatonin availability – key regulators of circadian rhythms. The gastrointestinal tract also produces melatonin at levels hundreds of times higher than plasma, indicating significance in sleep–wake regulation.
Across sleep disorders, common microbial trends emerge. Large cohort studies of chronic insomnia reveal reduced Ruminococcaceae levels and links between bacterial changes, bile-acid profiles and cardiometabolic disease risk. Obstructive sleep apnoea is associated with decreased bacterial diversity and distinct shifts in gut composition, reproduced in animal models exposed to intermittent hypoxia. Circadian rhythm disruption in shift workers corresponds with increased Actinobacteria and Firmicutes, alongside inflammatory changes, suggesting that microbiota adapt to – and exacerbate – misaligned sleep cycles.
Microbial signatures also appear in narcolepsy and rapid-eye-movement (REM) behaviour disorder, both of which display altered gut communities correlated with symptom severity. Reduced Faecalibacterium and Butyricicoccus levels may signal progression from REM behaviour disorder to Parkinson’s disease, offering a potential biomarker for neurodegeneration.
The review further highlights links between gut microbiota, sleep and mental health. Altered microbial communities occur in depression, anxiety, autism and Parkinson’s disease, often alongside disturbed sleep. In major depressive disorder, genera such as Blautia and Coprococcus correlate with poorer sleep, while children with autism and insomnia show decreased Faecalibacterium and altered serotonin–melatonin balance. In Parkinson’s disease, distinct gut profiles – particularly increases in Escherichia coli and Akkermansia muciniphila – differentiate those who experience early non-motor symptoms such as insomnia.
Building on these insights, the review evaluates microbiota-targeted therapies. Probiotics, live microorganisms that confer health benefits, have improved sleep quality and cortisol levels in trials involving patients with chronic insomnia, Parkinson’s disease and substance-use disorders. Lactobacillus plantarum PS128, for instance, increased restorative N3 sleep, while Bifidobacterium breve CCFM1025 reduced cortisol and improved subjective sleep quality. Animal studies reinforce these results, linking probiotic supplementation with longer non-rapid-eye-movement sleep and reduced anxiety-like behaviour.
Prebiotics – dietary substrates that support beneficial bacteria – also improve sleep metrics. Trials with guar gum and resistant dextrin show enhanced sleep quality and reduced inflammation. In rodents, prebiotic diets accelerate circadian realignment and recovery after sleep loss, possibly by modulating metabolites such as Parabacteroides distasonis. Synbiotics, which combine probiotics and prebiotics, offer additive effects; studies in post-acute COVID-19 syndrome show reduced insomnia severity and improved sleep scores after eight weeks of treatment.
Faecal-microbiota transplantation (FMT), the process of transferring gut microbes from healthy donors to recipient patients, represents a more radical but promising therapy. Small studies report significant improvements in insomnia severity and sleep quality, alongside rises in beneficial bacterial populations. Trials involving fibromyalgia, autism and long-COVID patients show comparable outcomes, suggesting broader therapeutic potential.
Each approach, however, carries limitations. Probiotics and prebiotics are safe and accessible for general use, while FMT remains restricted to research due to safety and regulatory challenges such as donor screening and infection risk. Synbiotics may offer the best balance of efficacy and practicality through combined microbial and metabolic support.
To advance the field, the authors propose a four-stage research framework. The first stage aims to correlate microbiome data with sleep measures using neuroimaging, polysomnography and metabolomics. The second seeks to identify predictive biomarkers through multi-omics and machine-learning integration. The third focuses on causal studies using FMT and controlled interventions. The final stage calls for large, standardised randomised trials assessing specific microbial therapies and their molecular effects on sleep physiology.
“While significant progress has been made, important challenges remain. We need larger, well-controlled clinical trials with standardised methodologies to validate therapeutic approaches and understand individual-response variability,” Professor Lu stated.
This review establishes the microbiota–gut–brain axis as a vital but underappreciated regulator of sleep. Evidence across disorders indicates that gut dysbiosis both contributes to and results from poor sleep, reinforcing pathological cycles of inflammation and metabolic disruption. Common patterns – such as increased Firmicutes/Bacteroidetes ratios and decreased Bacteroides, Bifidobacterium and Faecalibacterium – suggest shared microbial signatures underlying diverse sleep conditions.
As research progresses, microbiota-based strategies may transform how clinicians manage sleep disorders. Precision probiotics, optimised prebiotics and personalised synbiotics tailored to microbial profiles could form the basis of next-generation treatments aimed not only at improving sleep but also at enhancing neurological and metabolic health. A deeper understanding of the gut–brain connection may ultimately redefine sleep medicine and its role in overall wellbeing.
For further reading please visit: 10.61373/bm025i.0128
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh