Research news
FDA approves elinzanetant following UK’s MHRA first-in-world marketing authorisation
Oct 28 2025
A novel drug to relieve menopausal hot flushes, night sweats and sleep disturbance has gained approval from the United States Food and Drug Administration, offering a non-hormonal alternative for millions of women who cannot, or prefer not to, take oestrogen-based treatments
The US Food and Drug Administration (FDA) has approved elinzanetant, a novel non-hormonal therapy to relieve moderate to severe vasomotor symptoms such as hot flushes and night sweats in women experiencing menopause. The decision represents a major regulatory milestone and follows the earlier approval granted by the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom, which became the first authority in the world to license the treatment on 8 July 2025. The European Medicines Agency has not yet granted full marketing authorisation for the therapy.
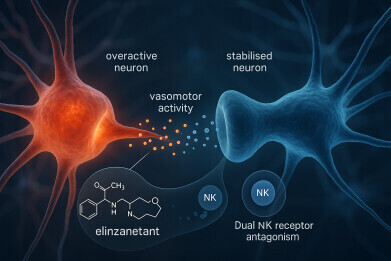
Elinzanetant – to be marketed with the brand name ‘Lynkuet’ – has been developed as an oral, once-daily capsule designed to alleviate the disruptive and often long-lasting symptoms associated with menopause. Unlike hormone replacement therapy (HRT), which until now has been the most effective intervention for vasomotor symptoms although it carries potential risks, elinzanetant contains no oestrogen or progesterone. Instead, it acts by targeting two neurokinin receptors in the brain that regulate temperature and mood, providing an alternative option for women who cannot – or prefer not to – take hormone-based therapies.
The FDA’s decision was informed by robust evidence from Bayer’s global phase III OASIS clinical programme, which included more than 1,400 post-menopausal women aged 40 to 65 drawn from countries across Europe, North America and Israel. Participants were randomly assigned to receive either a daily 120 mg capsule of elinzanetant or a placebo. The trials were conducted under double-blind conditions to ensure objectivity and statistical reliability.
Results from the OASIS I, II and III studies demonstrated that women treated with elinzanetant experienced a rapid and statistically significant reduction in both the frequency and intensity of hot flushes compared with placebo. Noticeable improvement was observed within the first week of treatment, with sustained benefit recorded over the 26 to 52 weeks of the study. Participants also reported improvements in sleep quality, mood and overall wellbeing. These findings were consistent across diverse demographic and clinical profiles, strengthening confidence in the reproducibility of the therapeutic effect.
The approval by the FDA comes amid growing recognition of the need for effective non-hormonal treatments to manage menopausal symptoms. Vasomotor symptoms are among the most common and distressing consequences of menopause, affecting more than one-third of women. Hot flushes and night sweats can persist for a decade or more after the end of menstruation, profoundly affecting quality of life, professional performance and sleep patterns. For some, these symptoms are accompanied by anxiety, irritability or depressive mood, exacerbating the overall burden of menopause.
Hormone replacement therapy remains the gold standard for treating such symptoms, as it directly replaces the hormones that decline during menopause. However, its use is often limited by safety considerations. Long-term HRT can increase the risk of blood clots, stroke and certain cancers. These risks have led many clinicians and regulatory authorities to emphasise the importance of developing safe, effective and non-hormonal alternatives.
By acting as a dual antagonist of neurokinin 1 (NK1) and neurokinin 3 (NK3) receptors, elinzanetant directly targets the neural circuits involved in temperature regulation within the hypothalamus. During menopause, declining oestrogen levels can disrupt the function of these neurons, narrowing the body’s thermoneutral zone and causing sudden heat surges and sweating episodes. Blocking the overactive neurokinin signalling restores equilibrium to the body’s internal thermostat, thereby reducing both the frequency and intensity of hot flushes.
Professor JoAnn V. Pinkerton, Director of Midlife Health at the University of Virginia Health and who was the US lead investigator for the OASIS II trial, described the approval as an important advance in women’s health.
“More than a third of women experience disruptive menopausal symptoms that can persist for more than a decade, significantly affecting work, home life and wellbeing. Many suffer without treatment or support.
“With the FDA’s approval of elinzanetant, women now have access to a safe and effective therapy for hot flushes and night sweats. Due to its dual receptor antagonism, the studies also showed improvements in sleep and mood,” she said.
When the MHRA became the first regulator to approve elinzanetant in July, it highlighted the significance of introducing a novel non-hormonal option for symptom relief.
“Hot flushes and night sweats associated with menopause can have a significant negative impact on quality of life. We are therefore pleased to announce our approval of elinzanetant, which has met the MHRA’s standards for safety, quality and effectiveness.
“Elinzanetant offers a non-hormonal alternative for those who may not be able to, or prefer not to, take hormone-based therapies. As with all licensed medicines, we will continue to monitor its safety closely as it becomes more widely used,” said Dr. Julian Beach, a director in Healthcare Quality and Access at the MHRA.
The clinical trial data also provided encouraging safety findings. Elinzanetant was well tolerated by participants, with headache and fatigue reported as the most common adverse effects. Both were generally mild and transient. No serious adverse reactions were recorded during the course of the studies. The safety profile supports its use as a long-term therapy for managing vasomotor symptoms, with minimal interference in patients’ daily activities.
The medicine is administered orally, taken once daily as a capsule. Its convenience of use is expected to support adherence among patients, particularly those who may be reluctant to pursue hormonal treatments requiring regular monitoring or who have previously discontinued HRT because of side effects or perceived risks.
The FDA’s decision builds upon growing regulatory and clinical momentum surrounding menopause care. Both approvals reflect an increasing recognition that the management of menopause extends beyond short-term symptom control to encompass quality of life, mental health and long-term wellbeing. With women spending more than a third of their lives in post-menopause, the need for evidence-based, safe and accessible therapies has become a major public health consideration.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh