Research news
Experimental ERK inhibitor shows promise in rare histiocytic cancers
Nov 07 2025
Memorial Sloan Kettering Cancer Center has reported promising early clinical results for ulixertinib, a novel extracellular signal-regulated kinase inhibitor that targets the mitogen-activated protein kinase pathway in histiocytosis. The therapy has improved outcomes for patients with Erdheim–Chester disease and related histiocytic neoplasms – a phase II trial is underway
An investigational therapy targeting the extracellular signal-regulated kinase (ERK) pathway has demonstrated significant clinical benefit for patients with histiocytosis, including the rare Erdheim–Chester disease, according to new findings from Memorial Sloan Kettering Cancer Center (MSK), New York.
Results from the first in-human study of the oral ERK inhibitor ulixertinib have shown that four out of five patients experienced measurable improvements in disease activity and quality of life. The phase I single-patient investigational new drug (IND) study was led by Dr Eli Diamond, who is a neurooncologist and early-phase drug development specialist at MSK. A multicentre phase II trial is now under way to assess efficacy in a larger cohort.
“The patients in this study were treated on what is called a single-patient IND application where we essentially write an individual clinical trial for each participant.” explained Dr Diamond.
“For rare diseases such as histiocytosis, this approach is a practical way to evaluate potential therapies when patient numbers are extremely small,” he said.
Histiocytosis comprises a group of uncommon disorders in which the body overproduces histiocytes – a type of white blood cell – that accumulate in tissues and organs to form tumour-like growths. The disease spectrum includes Erdheim–Chester disease, Langerhans cell histiocytosis and related entities.
Although collectively rare, these conditions can affect patients of any age and can cause extensive, multi-organ involvement with symptoms ranging from chronic fatigue and bone pain to neurological deficits and hormonal imbalance.
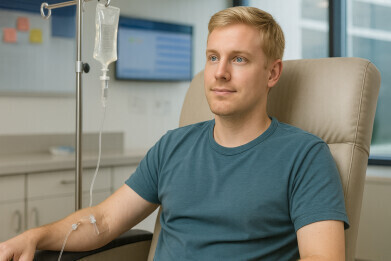
Dr Diamond’s patient, 34-year-old Joey Carlsen Martinez, had presented with severe fatigue, memory impairment and debilitating joint pain. He received ulixertinib under a compassionate-use authorisation at MSK. Within months, his symptoms had stabilised, and he has since regained mobility and resumed family life. His case illustrates the potential for targeted molecular therapy to transform outcomes even in ultra-rare malignancies.
Ulixertinib acts by inhibiting ERK, a critical downstream effector in the MAPK pathway. Dysregulation of this pathway is driven by mutations in protein-coding genes such as BRAF or MEK and underpins many histiocytic neoplasms and several other cancer types, including melanoma. Earlier therapies developed at MSK – notably vemurafenib (Zelboraf®) and cobimetinib (Cotellic®) – target upstream nodes in this signalling cascade but resistance or intolerance can limit durability of response.
“Drugs that block ERK have been studied in a number of cancers, but toxicity at higher doses has been a challenge,” Dr Diamond said.
“In histiocytosis, ulixertinib appears to act effectively at much lower doses, allowing patients to avoid most of the side effects seen in other settings,” he added.
The mechanistic understanding underpinning this progress has come from the laboratory of Dr Omar Abdel-Wahab, chair of the Molecular Pharmacology Program at the Sloan Kettering Institute, New York, and co-senior author of the study. Fifteen years ago, his group established the first genetically engineered mouse models of histiocytosis, enabling direct study of the disease’s molecular drivers.
These models, together with patient-derived samples sent to MSK from international collaborators, have informed the preclinical rationale for ERK inhibition and have guided the design of translational studies now reaching the clinic.
“MSK has a very active programme in histiocytosis, both in the laboratory and in the clinic.
“Our work in this field, and how it has ultimately benefited patients worldwide, demonstrates the importance of sustained investment in fundamental and translational research,” said Dr Abdel-Wahab.
The team’s findings suggest that ERK blockade could represent a third therapeutic class for histiocytosis, complementing existing BRAF and MEK inhibitors. Ongoing trials will determine the optimal dosing strategy and assess long-term disease control.
For patients such as Martinez, whose quality of life has markedly improved, ulixertinib has already made a tangible difference. He continues to receive treatment under the supervision of his care team in collaboration with MSK.
“I still tire easily, but I can cook, shop, and care for my family,” he said.
“Knowing how bad things once were, I am profoundly grateful for the care I’ve received.”
MSK’s leadership in histiocytosis research exemplifies how mechanistic insight can drive therapeutic innovation in rare disorders. The institution’s ongoing phase II trial of ulixertinib will determine whether ERK inhibition can become part of routine clinical management for these patients in the years ahead.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh