-

-
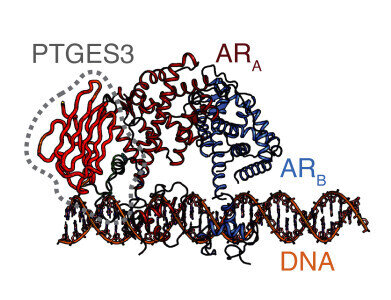 This figure shows the structure of PTGES3. Credit: Li et al.
This figure shows the structure of PTGES3. Credit: Li et al.
Research news
CRISPR screen uncovers hidden protein regulator key to understanding therapy-resistant prostate cancer
Nov 11 2025
A research team drawn from Arc Institute, the University of California San Francisco, and the Fred Hutchinson Cancer Center, Seattle, has shown that a previously overlooked protein plays a crucial role in driving aggressive prostate cancers, redefining the biological function of PTGES3 in hormone-resistant disease
A protein once considered to be an unremarkable chaperone or enzyme has been identified as a critical regulator of prostate cancer progression. Scientists from the Arc Institute, the University of California San Francisco (UCSF), and the Fred Hutchinson Cancer Center have used systematic CRISPR screening to reveal that the third prostaglandin E synthase protein (PTGES3) acts as an unexpected modulator of the androgen receptor. The discovery redefines the biological function of PTGES3 and highlights a promising therapeutic target for aggressive, treatment-resistant prostate cancers.
The androgen receptor is a hormone-sensitive protein that regulates prostate development and maintenance under normal conditions. However, in prostate cancer cells, its activity becomes excessively amplified, driving tumour growth and disease progression. While most existing treatments focus on inhibiting this receptor’s activity many tumours continue to evolve resistance.
To uncover which genes sustain androgen receptor activity, the researchers engineered prostate cancer cells with a fluorescent tag to track receptor levels in real time. Using genome-wide CRISPR screening, they disabled genes individually to determine which caused the fluorescent signal to diminish. This approach confirmed known androgen receptor regulators – including HOXB13 and GATA2 – thereby validating the screening method. Unexpectedly, PTGES3 also emerged as a significant regulator.
Since PTGES3 was the only one of three prostaglandin-synthesising enzymes to influence androgen receptor levels, the findings suggest that it may not act as a traditional enzyme. Rather, its role appears to be far broader.
“Our study illustrates the power of CRISPR approaches to take a quantitative, unbiased approach to discover something novel about a well-studied protein,” said senior author Dr. Luke Gilbert, core investigator at the Arc Institute and an associate professor of Urology at the UCSF School of Medicine.
“We were initially interested in identifying enzymes that might regulate androgen receptor biology because they are druggable, but we ended up with PTGES3, a protein that as far as we can tell is not an enzyme yet has a profound effect on the androgen receptor.”
Analysis of patient datasets revealed that individuals with high PTGES3 expression experienced markedly poorer outcomes when treated with hormone therapy. In mouse models, inhibition of PTGES3 slowed tumour growth and reduced androgen receptor levels, indicating that the protein could serve as a viable therapeutic target in hormone-refractory disease.
The research team found that PTGES3 operates through two mechanisms. In the cytoplasm, it functions as a co-chaperone that stabilises the androgen receptor protein, while within the nucleus it acts as a co-factor that facilitates receptor binding to DNA and activation of target genes. By supporting both receptor stability and gene regulation, PTGES3 may enable or even sustain tumour growth.
“Previous attempts to modulate transcription factor function for therapy have focused on DNA-binding and transcription activation domains.
“Targeting regulators of transcription factor stability, on the other hand, has received less attention,” said first author Haolong Li, who conducted the work at UCSF and is now an assistant professor at the Fred Hutchinson Cancer Center.
“Our study could serve as a template for understanding other transcription factors across different hormone-driven cancer types. Going forward, there are upwards of twenty transcription factors in oncology research that could benefit from this approach,” he added.
The researchers are now working to elucidate the structural mechanisms underpinning PTGES3’s interaction with the androgen receptor. Their long-term aim is to design therapeutics to disrupt this interaction, potentially through protein degradation technologies that are already showing promise in early-stage clinical trials.
The study was co-led by the late Professor Felix Feng, a leading figure in radiation oncology and translational cancer research at UCSF.
“We miss Felix deeply and hope this work is part of his legacy,” said Dr. Gilbert.
For further reading please visit: 10.1038/s41588-025-02388-8
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh