-
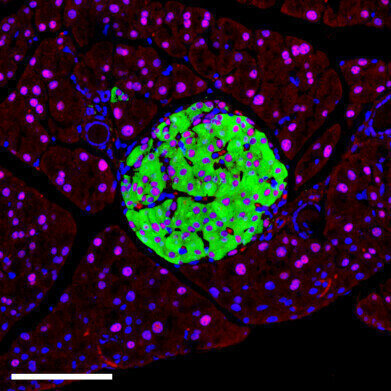 Picture: Insulin positive β cells (green) display HNF1A (red), confirming its presence across endocrine subtypes; DAPI (blue) marks nuclei. Credit: Miguel Angel Maestro Garriga/Centro de Regulación Genómica
Picture: Insulin positive β cells (green) display HNF1A (red), confirming its presence across endocrine subtypes; DAPI (blue) marks nuclei. Credit: Miguel Angel Maestro Garriga/Centro de Regulación Genómica
Research news
Single-gene linked to widespread type-2 diabetes via RNA disruption in insulin-producing cells
Aug 06 2025
Faulty genomic regulation reveals a new druggable foothold against T2DM and a rare MODY3 early onset form of the disease
Mutations in a single gene – HNF1A – have long been known to MODY3 diabetes, a rare, early-onset form of the disease. However, common smaller-scale mutations in the same gene have now been shown to predispose millions of individuals to the more common form of type-2 diabetes (T2DM), according to research published by a team at the Centre for Genomic Regulation in Barcelona
Researchers have revealed that HNF1A-linked diabetes arises from dysfunction in pancreatic β cells which produce insulin. The team used mouse models to switch off the HNF1A gene selectively in different tissues and cell types, including the liver, gut, and both α and β cells of the pancreas. Only deletion in β cells caused changes in blood glucose levels, demonstrating the gene’s critical role in insulin regulation.
HNF1A is a transcription factor that binds to DNA to modulate the activity of other genes. Its deletion in either human or mouse β cells affected the expression of more than 100 genes, many of which are essential for insulin transport and release. One of its direct genetic targets is A1CF – a second gene responsible for RNA splicing – the process by which precursor RNA is rearranged into mature sequences that can be translated into proteins.
The research showed that loss of HNF1A leads to a collapse in A1CF levels, resulting in widespread RNA splicing errors within the β cells. Between 1,900 and 2,300 RNA mis-splicing events were recorded.
“When HNF1A fails, two things go wrong at once. Hundreds of genes that depend on it begin to work incorrectly. That alone is enough to weaken insulin secretion, but the loss of A1CF means that the RNAs that are still made now get spliced incorrectly.
“Both layers matter, but the first hit is broader and sets the stage while the second piles on extra dysfunction,” said Matías Gonzalo De Vas, co-first author of the study.
The study also analysed human pancreatic tissue. In healthy donors, β cells displayed strong expression of HNF1A and A1CF. However, in donors with T2DM, most β cells exhibited low activity for both genes.
“In people with T2DM, for every high-functioning β cell we found about eight low-functioning ones, while healthy donors had a healthier ratio of one to one.
“It’s a dramatic shift that shows how a single mutation can cascade into the loss of function of entire tissues and organs,” said Edgar Bernardo, co-first author.
The study has identified a novel therapeutic entry point for both MODY3, which affects approximately 0.03% of the global population, and T2DM, which now affects more than one in nine adults worldwide – around 600 million people.
Therapies that correct RNA splicing errors have already shown clinical promise in other conditions such as spinal muscular atrophy. Because the diabetes defect identified here results from a splicing malfunction, a similar strategy to re-edit β cell RNA could potentially address a root cause of the disease.
“Existing therapies for diabetes try to lower blood sugar with different strategies without correcting underlying defects.
“The RNA defects we found are patchable, offering a rare, clear target for an incredibly complex disease,” explained Dr Jorge Ferrer, corresponding author of the study and researcher at the Centre for Genomic Regulation and CIBERDEM.
However, he cautioned against an overly simplified interpretation.
“We can now say this defective program has a causal contribution but there are other molecular defects that also need to be addressed. This is only one piece of a larger puzzle that we’ll also have to solve,” said Dr Ferrer.
His group now plans to build a molecular ‘parts list’ of the genetic hierarchy in β cells to identify the most actionable proteins and RNA molecules as future therapeutic targets.
“The goal is to pinpoint the most practical targets for new β cell therapies, so we can translate these insights into effective treatments,” concluded Dr Ferrer.
For further reading please visit: 10.1016/j.cmet.2025.07.007
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh