-
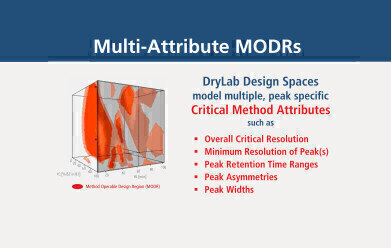 Fig. 1: Multi-attribute MODR including multiple criteria. Other than Rs,crit., further parameter requirements, like custom peak Rs, retention, asymmetry, width can also be addressed.
Fig. 1: Multi-attribute MODR including multiple criteria. Other than Rs,crit., further parameter requirements, like custom peak Rs, retention, asymmetry, width can also be addressed. -
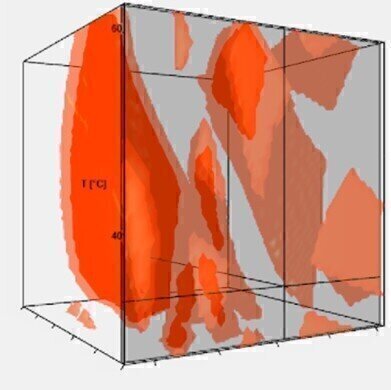 Fig. 2: Method Operable Design Region (MODR) of a 3D-model, displaying all suitable method parameter combinations yielding baseline separation
Fig. 2: Method Operable Design Region (MODR) of a 3D-model, displaying all suitable method parameter combinations yielding baseline separation -
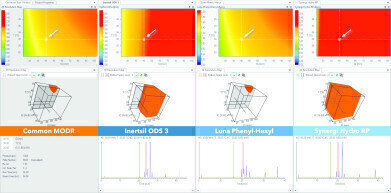 Fig. 3: Design Space Comparison of three single separation models acquired on stationary phases with different chemistries. On the left, the common, intercolumn MODR shows a small workable area where all three stationary phases are—in terms of resolving power—fully interchangeable. As seen however, at the currently selected point, only the Synergi Hydro RP column is delivering baseline separation while the other two would fail due to wrong method specification.
Fig. 3: Design Space Comparison of three single separation models acquired on stationary phases with different chemistries. On the left, the common, intercolumn MODR shows a small workable area where all three stationary phases are—in terms of resolving power—fully interchangeable. As seen however, at the currently selected point, only the Synergi Hydro RP column is delivering baseline separation while the other two would fail due to wrong method specification.
Laboratory products
DryLab Design Space Modeling in the Context of ICH Q14
Nov 01 2022
With what it introduces as the «enhanced approach», ICH’s Analytical Quality by Design guideline draft (Q14) promotes scientific analytical procedure outcomes, emphasizing knowledge-driven development, risk assessment and risk management. The approach requires more than the scope of what statistical tool-kits can deliver. Favorable instead is the combination of a chromatography-based design-of-experiments (DoE) and powerful mechanistic modeling. Such combination effectively establishes a comprehensive design space model: its inherent chromatographic knowledge space establishes and visualizes all parameter ranges that yield robust separations. In consequence, those established conditions offer maximum flexibility, both pre- and post-approval.
In both the industrial and academic context, DryLab Design Space Modeling is widely accepted for its alignment with the «enhanced approach» mentioned above, along with its ability to establish and visualize three-dimensional knowledge spaces that comprise more than a million possible workpoints of the modeled separation systems. First introduced in 1986, the software visualizes design spaces since 2008. While industry’s recent transition towards sophisticated method development strategies is mainly due to the paradigm shift heralded by the introduction of Q12 on pharmaceutical product lifecycle management, there are also business objectives for modeling and evaluating robustness of separations and separation systems on an everyday basis. Particularly DryLab’s Design Space Comparison and Automation Module are the tools that put these requirements into practice.
Recently, Design Space Modeling also finds application for the qualified characterization and comparison of individual separation systems. If—for instance—instrumentation, sample and studied method conditions remain identical, intercolumn design spaces can be built and differences between the modeled stationary phases can be evaluated holistically. Following this approach, DryLab Design Space Modeling delivers both scientific knowledge and systematic understanding and, at the same time, allows for the transfer of existing applications to new hardware or for the redesign of analytical methods altogether.
More so, DryLab models comprise multi-attribute options that allow to establish conditions that concurrently fulfill certain method-specific criteria. The resulting Method Operable Design Regions (MODRs) visualize those ranges, where critical method attributes for one or more peaks of interest are met. Figure 1 displays such an MODR within a design space as a combination of red, three-dimensional bodies. The red areas represent method conditions where pre-defined critical method attributes are fully met—attributes such as overall critical resolution (Rs,crit.) but also peak specific characteristics such as resolution (Rs), retention time windows (tR), peak tailing (Tf) and peak width(w).
Recent publications describe the comparison of design spaces to find column interchangeabilities, as well as the analysis of SARS-CoV-2 antibodies using DryLab’s Automation Module.
Design Space Modeling has become an important pilar of current analytical procedure development and soon expected to become even more demanded for real-time decision-making in prospective stages of data-driven pharmaceutical industry.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















