Laboratory products
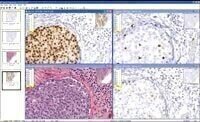
Fda Approval Received for Her2 Image Analysis Application
Apr 24 2008
intended to be used as an aid to pathologists in detecting and quantifying HER2 protein expression from digital slide images created by Aperio’s slide scanning systems.
Aperio’s FDA clearance encompasses the company’s complete digital pathology system, including ScanScope® scanners for creating digital slide images from microscope slides, the Spectrum™ digital pathology information management system for managing, viewing, and analysing
digital slides, and the specific image analysis application which performs the automated scoring of IHC HER2 breast cancer digital slides.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh