-
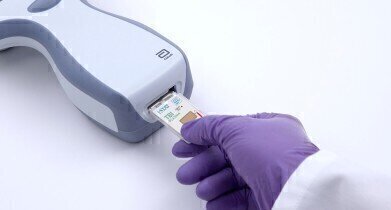 Abbott’s i-STAT TBI Plasma test will help clinicians assess individuals with suspected mild TBIs, including concussions.
Abbott’s i-STAT TBI Plasma test will help clinicians assess individuals with suspected mild TBIs, including concussions.
Laboratory products
Brain Injury Test Achieves CE Mark
Dec 15 2021
Abbott has achieved CE Mark for the i-STAT TBI Plasma test, the first rapid handheld traumatic brain injury (TBI) blood plasma test, which permits marketing in the European Union and will help clinicians assess individuals with suspected mild TBIs, including concussions. The test will run on Abbott’s handheld i-STAT™ Alinity™ platform. Test results are available in approximately 15 minutes after plasma is placed in the test cartridge.
The test measures specific proteins present in the blood after a mild TBI. A negative result on this test can be used to help rule out the need for a head CT scan, an imaging tool commonly used to diagnose concussion. For those who test positive, this test result complements CT scans to help clinicians in the further assessment of those patients who may have a mild TBI.
The i-STAT TBI Plasma test requires a small blood sample drawn from the arm, from which plasma is extracted with a centrifuge and applied to the test’s cartridge. The cartridge is then inserted into the handheld instrument.
Abbott is also working on a whole blood test, which would eliminate the need for separation of plasma and could be used at the patient’s side in a healthcare setting. Abbott’s vision for the future is to have a portable test that can be used outside the traditional healthcare setting where people experience head injuries and need a quick evaluation, like sporting events.
The i-STAT TBI Plasma test simultaneously measures glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1) in blood plasma, two complementary biomarkers that have been shown to be elevated after brain injury. It provides test results with 95.8% sensitivity and greater than 99% negative predictive value.
Abbott has also received CE Mark for a laboratory-based version of this test, which will help clinicians assess individuals with suspected mild TBIs, including concussions. The test will run on Abbott’s Alinity™ i laboratory instrument, which produces a result within 18 minutes.
Expanding use of the TBI blood test to Abbott’s Alinity i instrument will increase access to concussion assessment testing, as it is already used in major trauma centres across Europe.
When someone is sent for a CT scan to assess brain injury, the vast majority of results come back negative. This blood test can be used as an aid to rule out the need for a head CT scan, potentially reducing costs for patients as well as the amount of time they spend in the emergency department. Running on Abbott’s trusted Alinity i instrument, the laboratory test will provide a more objective and definitive method of concussion evaluation while enabling more rapid triaging of patients, offering increased confidence and efficiency for healthcare professionals.
“We know people may brush off a hit to the head for many reasons. Some are unaware of the signs and symptoms of concussion – and others are skeptical that a trip to the doctor will be able to give them a clear answer on whether they’ve injured their brains and what to do about it,” said Beth McQuiston, MD, Medical Director in Abbott’s diagnostics business. “This test is revolutionary because it provides an objective blood test result to aid in concussion assessment, so people can consult with their doctors to heal in the best way possible.”
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















