Lab safety
Genevac Solvent Evaporators: A Powerful Tool for Wearable Tech Research and Regulatory Compliance
Nov 19 2024
As wearable technology continues to advance, ensuring the safety and efficacy of these devices becomes increasingly important. One critical aspect of this is the evaluation of leachable and extractable substances (L&E), which are chemicals that can migrate from the device into the body. To address this, Genevac solvent evaporators have emerged as a valuable tool in research and development, offering reliable and efficient solvent evaporators for L&E analysis.
Understanding Leachables and Extractables
Leachables are substances that migrate from a device into the body during use. Extractables are substances that can be extracted from a device's materials under exaggerated conditions, representing a potential source of leachables. Both leachables and extractables can pose potential health risks, and their evaluation is crucial for ensuring the safety of wearable devices.
The Growing Need for L&E Analysis
Several factors contribute to the growing importance of L&E analysis in the wearable tech industry:
- Diverse Materials
- Direct Skin Contact
- Regulatory Scrutiny
- Consumer Awareness
Wearable devices often incorporate a variety of materials, including plastics, metals, adhesives, and coatings, each with its own potential L&E profile, and those such as smartwatches, smart rings and fitness trackers, have prolonged skin contact, increasing the potential for L&E migration. Regulatory bodies worldwide are increasingly focusing on the safety of wearable devices, including their potential to release harmful substances. Additionally, consumers are becoming more informed about product safety and are demanding transparency from manufacturers.
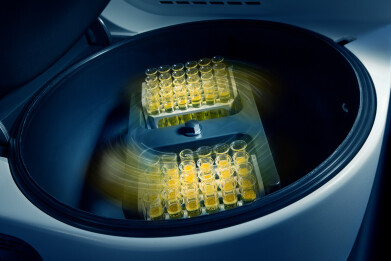
Genevac Solvent Evaporators: A Key Tool for L&E Analysis
Genevac solvent evaporators are designed to efficiently remove solvents from samples, making them ideal for preparing samples for L&E analysis. By rapidly evaporating solvents under controlled conditions, these evaporators help concentrate samples and eliminate potential interference from residual solvents, which can impact later analytical results.
Key features of Genevac solvent evaporators including, high efficiency, temperature control, anti-bumping technology, and versatility that make them ideal for L&E research as they offer accelerated solvent removal through centrifugal force and vacuum evaporation, precise temperature settings to prevent sample degradation, minimised sample loss and contamination, and able to handle a wide range of sample formats.
Regulatory Landscape for L&E Testing in Wearable Tech
Regulatory bodies worldwide have specific guidelines and standards for L&E testing in medical devices, including wearable technology. Some key regulations and standards include:
- FDA (Food and Drug Administration) specific regulations for medical and wearable devices. These regulations outline requirements for L&E testing, including the use of appropriate extraction solvents and analytical techniques.
- The ISO 10993 international standard series provides guidelines for the biological evaluation of medical devices. ISO 10993-18 specifically addresses chemical characterisation of medical device materials and extraction procedures.
- The EU Medical Device Regulation (MDR) sets stringent requirements for the safety and performance of medical and wearable devices with emphasis on the need for thorough risk assessment, which includes consideration of L&E.
By combining the power of Genevac solvent evaporators with these new technologies and adhering to regulatory guidelines, researchers and manufacturers can ensure the safety and quality of wearable devices, protecting consumers and promoting technological innovation in our day to day lives.
To discover more, contact Biopharma Group today (UK & Ireland)
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh

.jpg)

-(1).jpg)















