Chromatography
National Scientific Mass Spec Certified Vials
Nov 17 2010
Industry’s first and only pre-cleaned, low particle, low background chromatography vial When your instrumentation, sample handling and methodology is pushing the limits, a chromatography vial that can keep up is essential.
• The only chromatography vials pre-cleaned to provide unmatched consistency
• The first low particle, low background chromatography vials
• Pre-cleaned vial packaging protects the product integrity
• High purity closures packed in airtight re-closable container
• Tested and certified for 13 critical physical characteristics affecting vial performance
• Tested and certified for low background by positive ESI LC/MS
• Tested and certified for low background by GC/MS
Low Particle Background
The presence of inorganic sub micron particles in all glass vials as a by-product of the manufacturing process is a little known phenomenon that has not been extensively studied.
Gas chromatographers depend on injection port liners to act as traps for particulates while the HPLC chromatographer takes extensive steps to eliminate them during sample preparation.
This has been an effective strategy for routine analytical methods, but the need to work with ever lower concentrations of analytes creates the possibility of interactions with compounds of interest.
National Scientific Mass Spec Vials undergo a proprietary cleaning process that greatly reduces the background particulates and their potential affect on high sensitivity chromatography.
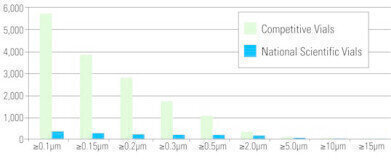
The table to the right gives a comparison of the particle distribution obtained from an analysis of standard vials versus the National Scientific Mass Spec Vials. All National Scientific Mass Spec Vials are processed and tested for background particulates.
A typical vial that has not been processed can exhibit particle counts exceeding 5000 particles per mL with the highest counts occurring in the range below 0.5μm.
This has traditionally been of little concern when GC inlet liners or HPLC guard columns are used. GC techniques employing on-column injection create the need for a sample vial with minimal background particulates to prevent an accumulation of foreign material at the head of the column than might adversely affect a separation. Similarly newer techniques employing finely packed HPLC columns, capillary columns and direct connection of the analytical column to the sample valve also require the elimination of as much particulate matter as possible from the sample stream.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh