-
 The illustration depicts a study by SLAC and Stanford, including cryo-EM imaging (left), that discovered how a cellular machine called TRiC (right) directs the folding of tubulin (yellow tangle at the center of TRiC). Tubulin is the protein building block of microtubules that serve as the scaffolding and transport system in human cells. The results challenge a 70-year-old theory of how proteins fold in our cells and have profound implications for treating diseases linked to protein misfolding. (Credit: Greg Stewart/SLAC National Accelerator Laboratory)
The illustration depicts a study by SLAC and Stanford, including cryo-EM imaging (left), that discovered how a cellular machine called TRiC (right) directs the folding of tubulin (yellow tangle at the center of TRiC). Tubulin is the protein building block of microtubules that serve as the scaffolding and transport system in human cells. The results challenge a 70-year-old theory of how proteins fold in our cells and have profound implications for treating diseases linked to protein misfolding. (Credit: Greg Stewart/SLAC National Accelerator Laboratory) -
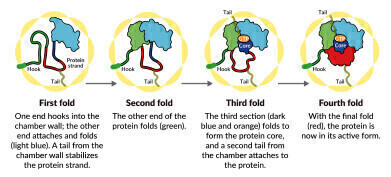 A SLAC-Stanford study revealed four intermediate steps in folding a human protein called tubulin, all directed by the inner walls of a cellular machine called TRiC (yellow). The process starts when a strand of tubulin enters the TRiC chamber. One end (green) hooks into the inner chamber wall; then the other end (light blue) attaches in another spot and folds, followed by the green end and two more folds of the middle sections (dark blue and red). The folding is directed by areas of electrostatic charge on the inner wall and by “tails” of protein dangling from the inner wall, which hold and stabilize the protein in the right configuration for the next step in folding. The protein core (dark blue) contains pockets (orange) where GTP, a molecule that stores and releases energy to power the cell’s work, plugs in. (Credit: Yanyan Zhao/Stanford University)
A SLAC-Stanford study revealed four intermediate steps in folding a human protein called tubulin, all directed by the inner walls of a cellular machine called TRiC (yellow). The process starts when a strand of tubulin enters the TRiC chamber. One end (green) hooks into the inner chamber wall; then the other end (light blue) attaches in another spot and folds, followed by the green end and two more folds of the middle sections (dark blue and red). The folding is directed by areas of electrostatic charge on the inner wall and by “tails” of protein dangling from the inner wall, which hold and stabilize the protein in the right configuration for the next step in folding. The protein core (dark blue) contains pockets (orange) where GTP, a molecule that stores and releases energy to power the cell’s work, plugs in. (Credit: Yanyan Zhao/Stanford University)
News
Landmark study reveals how TRiC nano chambers direct Protein Folding
Jan 19 2023
Scientists have challenged a 70-year-old theory of how proteins fold in our cells, having discovered how a tiny cellular machine called TRiC directs the folding of tubulin, a protein that is the building block of microtubules, which serve as the cell’s scaffolding and transport system in humans. The results of the study could have profound implications for treating diseases linked to protein misfolding, it was suggested.
Scientists have previously thought that while TRiC and similar machines known as chaperonins provide a passive environment conducive to folding, they were not directly involved in the process.
It has been estimated that up to 10% of the proteins in human, animal and plant cells are assisted with folding within nano-chambers into their final, active shapes. Many of the proteins that fold with the aid of TRiC are also intimately linked to human diseases, including certain cancers and neurodegenerative disorders like Parkinson's, Huntington’s and Alzheimer’s diseases, said Stanford Professor Judith Frydman, one of the study’s lead authors.
A lot of anti-cancer drugs, she added, are designed to disrupt tubulin and the microtubules it forms, which are really important for cell division. So targeting the TRiC-assisted tubulin folding process could provide an attractive anti-cancer strategy.
“This is the most exciting protein structure I have worked on in my 40-year career,” said SLAC/Stanford Professor Wah Chiu, a pioneer in developing and using cryogenic electron microscopy (cryo-EM) and director of SLAC’s cryo-EM and bioimaging division. “When I met Judith 20 years ago, we talked about whether we could see proteins folding. That’s something people have been trying to do for years and now we have done it.”
During the decade long study the researchers captured four distinct steps in the TRiC-directed folding process at near-atomic resolution with cryo-EM and confirmed what they saw with biochemical and biophysical analyses.
At the most basic level, Frydman said, this study solves the longstanding enigma of why tubulin can’t fold without TRiC’s assistance: “It really is a game changer in finally bringing a new way to understand how proteins fold in the human cell.
“Compared to the simpler folding chambers of chaperonins in bacteria, the TRiC in human cells is a very interesting and complicated machine,” Frydman said. “Each of its eight subunits has different properties and presents a distinct surface inside the chamber and this turns out to be really important.”
“These structural snapshots of intermediate stages in the folding sequence have never been seen before by cryo-electron microscopy,” Frydman said.
Due to the complexity of the analyses and the pandemic interlude, the study went on for so long that many of the people who worked on it have moved on to other jobs. They include postdoctoral researchers Daniel Gestaut and Miranda Collier from Frydman’s group, who carried out the biochemical part of the project and pushed it forward, and Yanyan Zhao, Soung-Hun Roh, Boxue Ma, and Greg Pintilie from Chiu’s group, who performed the cryo-EM analyses. Additional contributors included Junsun Park, a student in Roh’s group, and Alexander Leitner from ETH in Zurich, Switzerland.
The work was supported by grants to Wah Chiu and Judith Frydman from the NIH and grants to Soung-Hun Roh, who is now an assistant professor at Seoul National University, from the Korean National Research Foundation and Suh Kyungbae Foundation (SUHF).
More information online
Citation: Daniel Gestaut et al., Cell, 8 December 2022 (10.1016/j.cell.2022.11.014)
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh