-
 Enrica Pellegrino (Credit: Crick)
Enrica Pellegrino (Credit: Crick) -
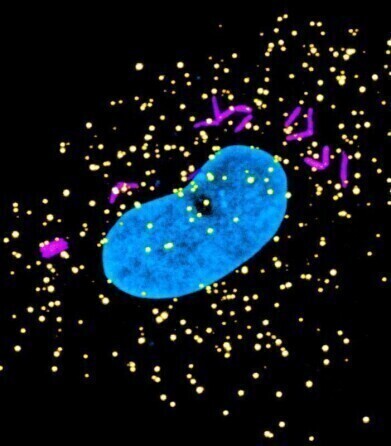 Microscope image of a human macrophage cell that is infected with tuberculosis bacteria (magenta), with the peroxisomes (yellow) and nucleus of the cell labelled (blue).
Microscope image of a human macrophage cell that is infected with tuberculosis bacteria (magenta), with the peroxisomes (yellow) and nucleus of the cell labelled (blue).
News
Research uncovers Immune Response to fighting TB Bacteria
Oct 12 2023
Researchers at the Crick have been able to show for the first time a mechanism that enables the human immune system to eliminate the bacteria that causes tuberculosis (TB), offering a new potential target for therapies against TB and other bacterial infections.
Peroxisomes, a small structure inside cells which make and break down fatty acids essential for metabolism, also generate by-products known as reactive oxygen species ROS. These highly reactive chemicals, such as hydrogen peroxide (H2O2), have also been found to help immune cells, such as macrophages, fight bacterial infections. In healthy conditions, cells can use ROS to regulate oxidative stress – a balance of these chemicals – in the cell. During infection, macrophages produce ROS when they engulf microbes in vesicles, or sacs, (phagosomes) which help to break down and kill the bacteria and are a key first-line defence for the immune system against infection.
One reason for the high infection rate of Mycobacterium tuberculosis (Mtb), - around 10 million people worldwide each year, which kills around 1.6 million of those infected - is thought to be the bacteria’s ability to damage the macrophage’s phagosome system, thus avoiding destruction and remaining in the water-soluble part of the cell, the cytosol, for long periods of time.
To investigate the specific mechanism of how macrophages fight back against this, the researchers used a combination of human stem-cell-derived macrophage (iPSDM) cells with fluorescent reporters to investigate the impact of peroxisomes on TB infection and ROS production, particularly hydrogen peroxide.
Presence of the TB bacteria was found to cause an increase in the number of peroxisomes in the cytosol of human macrophages and more peroxisomes had an altered shape.
After using CRISPR/Cas9 gene editing technology to delete genes responsible for peroxisome production, the researchers saw an increase in TB bacteria replication, suggesting that peroxisomes are crucial in restricting bacterial replication happening within the cell’s cytosol.
They tagged hydrogen peroxide with a fluorescent marker and saw that its levels increased in peroxisomes in cells infected with TB bacteria, but not in uninfected cells or in cells where bacteria couldn’t enter the cytosol.
These results suggest that human macrophages take advantage of hydrogen peroxide produced by peroxisomes to control the number of bacteria in the cytosol, even after these bacteria have been successful in evading the phagosome system.
The team then used drugs which boost peroxisome synthesis or increase hydrogen peroxide production in peroxisomes, which were found to restrict TB bacteria replication in the cytosol. This is independent of phagosome-associated pathways in macrophages, highlighting a back-up plan for cells to battle infection.
Enrica Pellegrino, first author and post-doctoral researcher at the Crick, said: “There’s not a lot of research done with mammalian cells, so we aimed to use both human macrophages and gene editing technology to understand in a human-relevant system how these cells can fight infection when bacteria outsmart the phagosome degradation pathways. Understanding these pathways may help us develop host-targeted therapies against infection, not only against TB but also other bacteria that can access the cytosol of infected cells.”
Max Gutierrez, Group Leader of the Host-Pathogen Interactions in Tuberculosis Laboratory at the Crick, said: “Our study brings another layer of control in the development of an immune response against intracellular bacteria and highlight that our immune cells have multiple mechanisms to restrict the spreading of infections.”
The next steps for the research will focus on investigating if this new mechanism applies to infections with other types of bacteria, with the hope that this opens up new potential avenues for antimicrobial therapies.
The study was published in the Journal of Cell Biology
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















