-

-
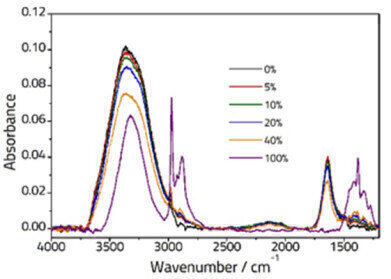 Figure 1: Spectra showing various concentrations of Ethanol/Water solutions recorded on an ArrowTM ATR Slide.
Figure 1: Spectra showing various concentrations of Ethanol/Water solutions recorded on an ArrowTM ATR Slide. -
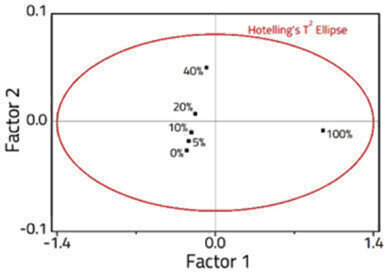 Figure 2: PLS Scores plot showing the sample distribution in 2 -factor space.
Figure 2: PLS Scores plot showing the sample distribution in 2 -factor space. -
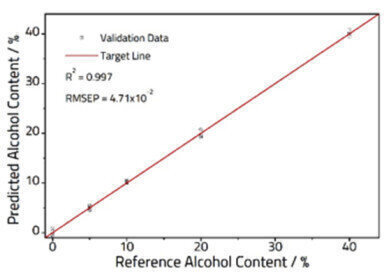 Figure 3: Calibration plot comparing known and PLS predicted alcohol content.
Figure 3: Calibration plot comparing known and PLS predicted alcohol content.
Mass spectrometry & spectroscopy
New Consumable ATR Solution Revolutionises Batch Testing of Samples in the Food and Drink Industry
Oct 29 2020
Arrow™ is a revolutionary new consumable ATR solution with applications ranging from testing of chemically aggressive samples through to large scale batch testing of liquid samples and solids dried from solution. Arrow consists of a thin Si ATR element fixed into a plastic slide for mounting on the Quest™ ATR unit. It also enables the slides to be conveniently handled and stored without coming into contact with the sample area. The slide is made from 100% recycled polypropylene.
Here we demonstrate the suitability of Arrow for batch testing of samples in the food and drink industry.
Accurate quantification of the composition of food and drink is essential for compliance with local regulations on labelling of goods for general sale and for correct application of customs duties when imported. Food and drink labelling should provide correct information about calorific and nutritional content to help people stay within recommended limits.
Solutions of water/ethanol in 26 different concentrations were prepared. A batch of Arrow™ slides were filled with aliquots from each solution and then covered with the Arrow™ sample cap to prevent evaporation. One slide was retained for use as a background. The prepared batch was then transported to the spectrometer for analysis.
Spectra were recorded on a commercially available spectrometer. The background spectrum was recorded first, followed by each of the 26 Arrow™ sample slides in turn. The complete procedure takes less than 10 minutes because there is no wipe down and cleaning of the sampling point is required between each spectral acquisition.
Figure 1 shows selected spectra for different concentrations of Ethanol solution. The pure water spectrum (black line) shows characteristic peaks at ca. 3350 cm-1 (assigned to two overlapped peaks: symmetric, ν1, and antisymmetric, ν3 stretching vibrations) and ca.1640 cm-1 (assigned to the ν2 bending vibration). The pure spectrum of ethanol (purple line) displays peaks due to an OH stretching vibration at ca. 3330 cm-1, CH stretching vibrations typical of an alkyl group between 3000-2800 cm-1 and further peaks in the fingerprint region below 1500 cm-1. The spectra of the mixture samples are a combination of the two pure samples, however due to interactions between the two some small changes are observed.
A 2-Factor PLS model was constructed from the spectra (Figure 2), explaining 99.9% of the variance within the dataset. The model was validated using fresh samples (Figure 3), and an excellent correlation was observed with an R2 = 0.997, and a RMSEP = 4.71 x 10-1.
This demonstrates that Arrow™ can be used to successfully quantify alcohol concentrations in a simple model system using PLS and identify all relevant spectral bands. For applications requiring the bulk testing and spectral discrimination of more complex systems such adulterated or contaminated foodstuffs, the speed of spectral acquisition and convenience of batch sample preparation come into their own.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















