Mass spectrometry & spectroscopy
Find out how the HT/HP cell has been used to investigate the impact of heating coal samples on its oxygen content.
Jun 14 2021
Introduction
In-situ analysis of materials at high temperature and pressure using infrared spectroscopy has become an indispensable method in modern material characterisation. Traditional methods are often not capable of obtaining satisfactory sample spectra of dark materials such as coal and graphite, where the main difficulties stem from poor light throughput, sample processing, data analysis, and baseline shifting.
Coal is produced over millions of years from underground sediments subject to heat and pressure, in a process known as coalification. The resulting coals are ranked according to their total carbon content and heating value. When analysing coal samples using Specac’s tri-modal High Temperature High Pressure cell (HT/HP cell) in conjunction with FTIR, it quantitatively measures chemical composition and the extent of deterioration through absorbance change during oxidation at different temperatures. The absorbance changes to oxygenated functional groups, aliphatic C-H groups, and aromatic C-H groups are of particular interest when monitoring coal oxidation, commonly referred to as combustion. Coal’s functional groups can be used to determine its storage, autoignition, and combustion properties, and ultimately its quality and price.
Experimental
1.5-2.0 mg of coal sample was weighed and dried under vacuum at 105ºC for two hours to remove moisture. The coal sample was then mixed with potassium bromide (KBr) powder and pressed into a pellet for analysis. For FTIR measurement, the sample was placed into the Specac HT/HP cell, which was then filled with a nitrogen atmosphere. Spectra were recorded in the 4000-500 cm-1 range with a resolution of 4 cm-1 and 32 scans on a commercially available spectrometer.
Results and Discussion
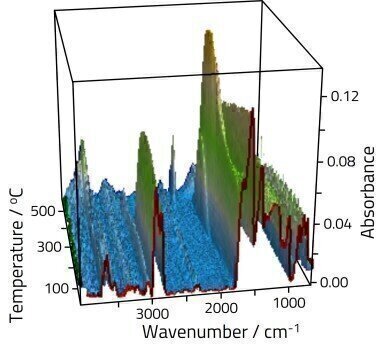
Coal contains a complex mixture of organic and inorganic species. Organic oxygenated functional groups found in coal include hydroxyls, carbonyls, ether, quinone rings, and other oxygenated heterocycles. These functional groups form complex bonds and display unique vibrational modes; some of these groups absorb infrared beam in the ~1300-1000 cm-1 region. A small percentage of the oxygen content of coal exists in carboxyl groups; the majority is found in hydroxyl groups and other oxygenates; the oxygen content in the latter contributes to half of the total oxygen content in coal.
In Figure 1, two sharp absorption bands at 2900 cm-1 and 2800 cm-1 are assigned to saturated aliphatic C-H; additionally a weaker band at 3050 cm-1 is attributed to unsaturated aromatic C-H. These bands are relatively stable as the temperature is increased up to about 400ºC, before rapidly decaying. Changes are also obvious in the fingerprint region, although assignment here is complex due to multiple overlapping features. At lower temperatures aromatic groups dominate the spectra in the 1550-1700 cm-1 region. As the sample is heated features grow into the high energy side of these peaks assigned to oxygenate materials.
Read the rest of the application note.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh