Laboratory products
Cutting-edge solutions for precision and sterility in aseptic fill-finish
Feb 12 2024
Increased demand for injectable drugs and stricter regulations drive surge in aseptic fill-finish technology, with Biopharma Group emerging as a trusted partner for UK and Irish pharma companies.
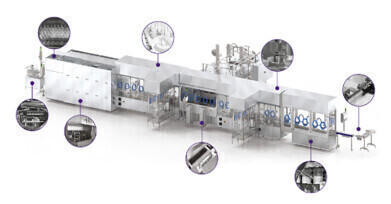
Why is aseptic fill-finish critical? This process safeguards the efficacy, integrity, and ultimately, the safety of sterile medications. With the UK and Ireland's aseptic fill-finish market projected to reach £3.8 billion by 2025, Biopharma Group highlights the importance of:
- Precision: Choosing the right equipment for accurate dosing and sterility is paramount
- Compliance: Modern systems prioritise features like validated cleaning, data integrity, and container closure integrity testing
- Patient Safety: Integrated cleanroom environments, advanced sterilisation technologies, and automated inspection systems minimise contamination risks
Case Study: A leading pharmaceutical company reduced waste, increased efficiency, and improved product quality with Biopharma Group's aseptic fill-finish solutions. The results were very impressive:
- 50% reduction in waste product
- 20% increase in production efficiency
- 30% reduction in downtime
- Significant improvement in product quality observed
- Clear return on investment
Biopharma Group's Value Proposition:
- Partnerships: Collaborating with ATS Scientific Life Sciences, Biopharma Group offers a comprehensive equipment range
- Expertise: 35 years of experience in the UK and Irish markets
- Solutions:
- Aseptic fill-finish equipment: Isolators, RABS, filling lines, inspection systems
- Freeze dryers and lyo services: Expertise in lyophilisation with advanced freeze dryers and validated processes
- Technical support and training: Comprehensive support throughout the process
Elevate your injectable drug production with Biopharma Group's commitment to precision and sterility in aseptic fill-finish.
Contact Biopharma Group today to discuss your requirements.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh