Laboratory products
Improved Workflow and Data Integrity - RFM900-T Series Refractometers
Apr 21 2021
Xylem’s Bellingham + Stanley brand RFM970-T refractometers have already established themselves as the ultimate refractometer for use in chemical and pharmaceutical industries with users already benefiting from the instrument’s wide scale range (1.30-1.70 RI), high precision (up to 6 decimals RI), compliance with FDA Regulation 21 CFR Part 11, as well as its durability. Now, recent upgrades to both hardware and onboard software allows users to take their laboratory to an even higher level.
New Network User Authentication synchronises passwords from the user’s company server, eliminating the need for duplicate login control within the instrument itself. Our FTP Instrument Sync - CLI Software further secures laboratory data by making use of Windows® Task Scheduler to automatically copy or move encrypted files from the refractometer to a local PC or company server at a defined period.
Additionally, customisation of the instrument’s touchscreen display is possible. Users may install and assign iconography to the instrument’s Method display to make selection easier. The boot-up splash screen and screensaver can also be personalised to reflect the environment in which the instruments operates; perhaps with a company logo, local help contact information or a GLP informational notice. Importantly, report headers may be customised with a company logo and site addresses so that they conform to branding guidelines and provide for a better audit trail.
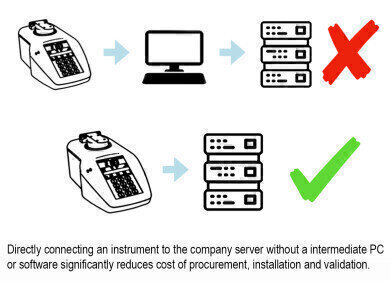
These latest features augment the previously released FDA reg. 21 CFR Part 11 enhancements. FDA reg. 21 CFR part 11 states that closed computer systems must have a collection of technological and procedural controls to protect data within the system. The RFM970-T refractometer meets this requirement without the need for an additional PC or supplementary software and in so doing, procurement, validation and maintenance costs are significantly reduced.
The recording of results, and the logging of instrument access and configuration is also possible. The Review & Approve electronic signature process can even be achieved locally or remotely, no matter where in the world. Encrypted Print to Secure PDF, .csv and .XML outputs makes data integration to LIMS simple, secure and auditable, especially when using the new CLI Software previously mentioned.
These new features, along with others such as synchronised server clock, Mean Method for batch analysis, Method limit check and maintenance prompts are not only available in the RFM970-T refractometer but are common to a refined selection instruments such as the high precision ADP600 Series of multiple wavelength, Peltier controlled polarimeters. A collection of instruments to choose from that helps your laboratory comply easily with FDA reg. 21 CFR Part 11 as well as other Data Integrity requirements or norms within the pharmaceutical market such as European Pharmacopeia 2.2.6 for refractometry and 9.9.5 for polarimetry.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh