-
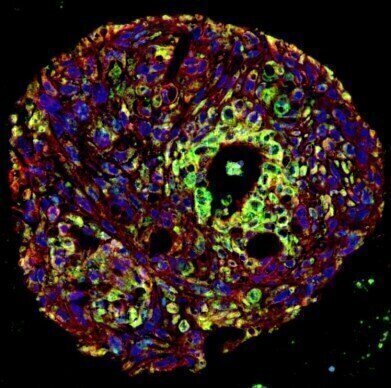 Human bronchial organoids infected with SARS-CoV-2 after cryopreservation in STEM-CELLBANKER®. Red: acetylated α-tubulin, green: SARS-CoV-2-N protein, blue: DAPI. Images courtesy of Kazuo Takayama (CiRA, Kyoto University, Japan).
Human bronchial organoids infected with SARS-CoV-2 after cryopreservation in STEM-CELLBANKER®. Red: acetylated α-tubulin, green: SARS-CoV-2-N protein, blue: DAPI. Images courtesy of Kazuo Takayama (CiRA, Kyoto University, Japan). -
 GMP grade STEM-CELLBANKER cryopreservation media.
GMP grade STEM-CELLBANKER cryopreservation media.
Laboratory products
GMP-compliant Products for Pre-clinical and ATMP Research
Sep 26 2022
High quality GMP-compliant products and services
Life science and healthcare specialists, AMSBIO, reports upon its extensive range of high quality GMP-compliant products and services for pre-clinical and advanced therapy medical products (ATMP) research.
The importance of GMP compliance in Regenerative Medicine research
Good Manufacturing Practice (GMP) is an internationally recognised system which ensures that products are consistently manufactured to a high-quality and provides key guidelines for the manufacture of Advanced Therapy Medicinal Products (ATMPs). Operating as an ISO 9001 registered organisation, AMSBIO understand the importance of offering GMP compliant products and services with guaranteed quality and consistency for your pre-clinical and clinical ATMP research.
Visitors to amsbio.com can learn about, select and order from AMSBIO’s extensive range of GMP compliant cell culture media, recombinant proteins, dissociation enzymes, cryopreservation, and viral gene delivery products. Sourcing from AMSBIO you can be assured of the consistent, high-quality of their GMP-compliant materials and in your supply chain. Critically to ensure the traceability of all their GMP compliant products, AMSBIO can also supply associated FDA-registered Drug Master Files and provide ICH stability study data supporting enzyme storage and shipping conditions. Drawing upon its experience of serving pre-clinical and clinical ATMP research, AMSBIO also offers high quality, GMP-compliant custom product development services for numerous research areas.
Download the AMSBIO ‘Guide to GMP’ to learn more about Good Manufacturing Practice.
Contact AMSBIO to learn about GMP compliant products and services to streamline your clinical translation.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















