-
 Figure 1: Efficient separation and analysis of antibody fragments and their drug conjugates.
Figure 1: Efficient separation and analysis of antibody fragments and their drug conjugates. -
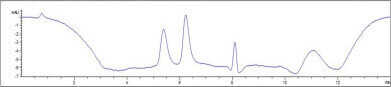 Figure 2: Chromatogram from the analysis of the mixture of an unconjugated protein scFv and the conjugated protein FDC using 75 mM ammonium acetate/ 4.5% 2-propanol as eluent.
Figure 2: Chromatogram from the analysis of the mixture of an unconjugated protein scFv and the conjugated protein FDC using 75 mM ammonium acetate/ 4.5% 2-propanol as eluent. -
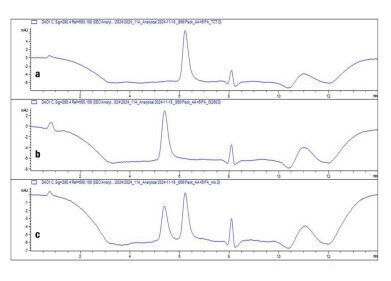 Figure 3: Chromatograms from the analysis of the unconjugated protein scFv (a), the conjugated protein FDC (b) and a mixture of both (c) using 75 mM ammonium acetate/ 4.5% 2-propanol as eluent.
Figure 3: Chromatograms from the analysis of the unconjugated protein scFv (a), the conjugated protein FDC (b) and a mixture of both (c) using 75 mM ammonium acetate/ 4.5% 2-propanol as eluent.
Columns
Efficient separation and analysis of antibody fragments and their drug conjugates
Apr 10 2025
Alongside monoclonal antibodies (mAbs) and antibody-drug-conjugates (ADCs) antibody fragments and their drug conjugates find application in therapeutic treatments. These fragments bind selectively to specific antigens, mirroring the function of intact antibodies. Due to their significantly smaller size, antibody fragments require dedicated analytical methods for precise characterisation and quality control.
This Application Note presents a precise method to separate a single-chain variable fragment (scFv) from its conjugated species (FDC). The separation was performed using a SEC-MS method. To achieve optimal resolution and peak shapes, a bioinert column hardware is essential. A metal-free PEEK-lined YMC-Pack Diol-120 column was selected for this analysis, ensuring high performance in the characterisation of the scFv with a molecular weight of approximately 26 kDa.
The separation of the scFv and FDC, injected individually and as a mixture, each at a concentration of 0.5 mg/mL, without isopropanol in the mobile phase already achieved sufficient resolution. But the target FDC-to-scFv ratio, based on peak area, is approximately 0.8. This ratio is not achieved under these conditions, either for individual injections or for the mixture.
The addition of 4.5% isopropanol to the mobile phase increases the peak area of the hydrophobic FDC, allowing the ratio of 0.8 to be reached in both cases (Figure 2). Additionally, isopropanol improves peak symmetry, further enhancing separation performance.
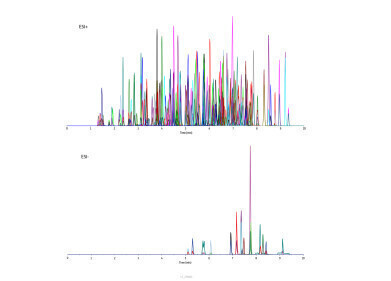
The deconvoluted mass spectra of the scFv and FDC confirm the separation of both compounds. That makes this method an ideal tool to monitor the conjugation of the scFv.
Download the Application Note for full method details.
*Application data by courtesy of Laura Bouché and Anja Pomowski, Antikor, Stevenage, UK.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh