Chromatography
Nitrite analysis in pharmaceuticals: monitoring a precursor to nitrosamine
Feb 09 2024
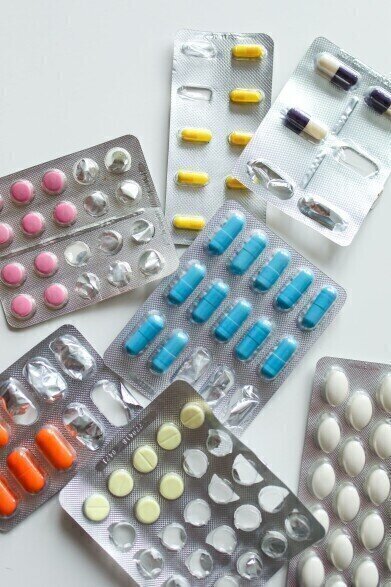
Nitrosamines are carcinogenic, even at trace levels. As a result, the FDA issued guidance regarding the monitoring of nitrosamine impurities in drugs made for human consumption. Monitoring nitrite impurities in pharmaceutical products and raw materials can be part of a control strategy for nitrosamines. Ion chromatography (IC) is well suited for the analysis of trace amounts of nitrite.
IC is an excellent method for measuring trace levels of nitrite. Unlike other testing methods, chloride does not interfere with an IC’s analysis of nitrite. Sample preparation through solid phase extraction or pre-derivatisation is therefore not necessary with IC. Additionally, IC can simultaneously detect multiple ionic impurities in drug products.
A Metrosep A Supp 10 column with UV/VIS detection is the preferred choice for the IC analysis of nitrite in pharmaceutical industry. Inline sample preparation techniques, such as the Metrohm intelligent Preconcentration Technique with Matrix Elimination (MiPCT-ME), further increase the sensitivity and robustness of the analysis. Moreover, preconcentrating the sample increases the sensitivity, allowing even trace amounts of nitrite to be detected. Matrix elimination improves the robustness by removing the interfering sample matrix.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh