-
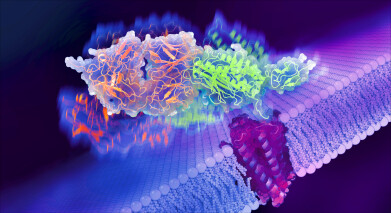 Adhesion G protein-coupled receptors have a large extracellular region (shown in green and orange) that extends into space outside the cell. Picture credit: Eric Smith, UChicago
Adhesion G protein-coupled receptors have a large extracellular region (shown in green and orange) that extends into space outside the cell. Picture credit: Eric Smith, UChicago
Research News
Detailed images of crucial cell receptors show promising targets for drug discovery
Dec 27 2024
Researchers at the University of Chicago have captured complete images of adhesion G protein-coupled receptors and an alternate means of activating them, opening new opportunities for drug design
G protein-coupled receptors (GPCRs), proteins embedded in cell membranes, are the target for almost 35% of all drugs that have been approved by the Food and Drug Administration. By comparison, adhesion G protein-coupled receptors (aGPCRs) which form the second largest family of these receptors in humans have no drugs approved to target them.
The aGPCRs receptors are involved in processes such as tissue growth, immune response and organ formation. When aGPCRs malfunction, they play a role in the development of cancers, brain disorders and contribute to growth issues, as well as other diseases.
Research from the University of Chicago (UChicago) combined two powerful imaging techniques to study the complete structure of a common aGPCR, including how the long and complex extracellular region it presents (see picture) interact with the transmembrane region embedded in a cell’s surface. The different positions and movements of the extracellular region appear to be an important way to activate the receptor.
“This opens up new opportunities for drugging adhesion GPCRs, because … the extracellular region is communicating with the transmembrane region,” said Dr Demet Araç, associate professor of biochemistry and molecular biology at UChicago and senior author of the study published in Nature Communications.
The extracellular region of an aGPCR extends from the cell membrane into space outside the cell, where it can bind to molecules and receptors from other cells. It consists of several domains, including the GPCR Autoproteolysis INducing (GAIN) domain, which can cleave itself into two pieces.
It was believed that aGPCRs were activated by a ligand from outside the cell attaching to one of the extracellular domains and splitting the GAIN domain, leaving a peptide from this domain called the tethered agonist (TA) attached to the transmembrane region. This separated TA can move and interact with the transmembrane region to initiate signalling.
But a growing body of biochemistry research shows that many aGPCR functions don’t rely on this cleavage-dependent mechanism. Separating the GAIN domain is also irreversible, leaving the receptor in a constant “on” state, which may be harmful for the cell. A cell may need to toggle a receptor on and off, so there must be some other way of doing it.
Araç’s lab has been working for more than a decade to reveal the structure of full-length aGPCRs, hoping to learn how incoming signals are transmitted from outside to inside the cell.
Graduate student Dr Szymon Kordon led the new study further developing earlier work to capture images of the complete structure of Latrophilin3, an aGPCR involved in developing brain synapses that has also been linked to both attention deficit hyperactivity disorder and some cancers.
Kordon and Araç optimised generation and purification of Latrophilin3 and captured initial electron microscopy images, but they faced numerous challenges to get a good picture of the receptor.
Working with Dr Antony Kossiakoff, the Otho S.A. Sprague Distinguished Service Professor of Biochemistry and Molecular Biology at UChicago, to create a synthetic antibody that could attach to the aGPCR. The extracellular region was stabilised by this antibody and gave it a distinctive shape allowing Kordon to capture an image of the full receptor structure by using cryo-electron microscopy (cryo-EM). The resulting images were the first to capture the structure of a complete aGPCR.
The cryo-EM images showed that the GAIN domain of the receptor assumed several different positions in relation to the cell surface. Each different position of the GAIN domain created a different contact point between it and the transmembrane region. The researchers wondered if these different configurations could be a different means of communicating to the cell, without separating the GAIN domain completely. So, they partnered with Dr. Reza Vafabakhsh, associate professor of molecular biosciences at Northwestern University, and Dr Kristina Cechova, a postdoctoral researcher at Northwestern, to run a second series of experiments that tracked the movements of the extracellular regions.
Cechova and the team used Förster resonance energy transfer (FRET) imaging, which can measure the energy transfer between molecules that are close to each other. After attaching fluorescent markers to different points on both the extracellular and transmembrane regions of the aGPCR, they could track its movements as it responded to adhesion forces pulling and pushing on it. What they saw confirmed their suspicions about the function of the different configurations.
The study, “Conformational coupling between extracellular and transmembrane domains modulates holo-adhesion GPCR function,” was supported by the National Institutes of Health, the Chicago Biomedical Consortium, and the National Cancer Institute. Additional authors include Sumit J. Bandekar, Katherine Leon, and Przemysław Dutka from UChicago and Gracie Siffer from Northwestern.
To read more visit: 10.1038/s41467-024-54836-4
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan