-
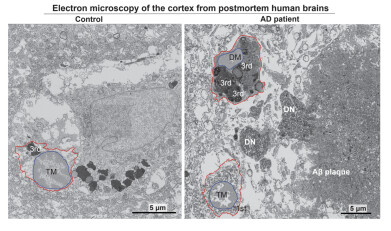 Electron micrographs show typical microglia in the prefrontal cortex of a 92-year-old healthy female (left) and dark microglia a 91-year-old female patient with Alzheimer’s disease (right). Picture credit: Anna Flury, CUNY
Electron micrographs show typical microglia in the prefrontal cortex of a 92-year-old healthy female (left) and dark microglia a 91-year-old female patient with Alzheimer’s disease (right). Picture credit: Anna Flury, CUNY
Research News
Researchers identify key cellular mechanism driving progression of Alzheimer’s disease
Dec 23 2024
Breakthrough marks a promising target for novel drug therapies that could slow development of the disease
Researchers at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC), part of the City University of New York, USA have discovered a critical mechanism that links cellular stress in the brain to the progression of Alzheimer’s disease (AD).
The study, published in the journal Neuron, highlights microglia, the brain's primary immune cells, as central players in both the protective and harmful responses associated with the disease.
Microglia, often dubbed the brain's first responders, are now recognised as a significant causal cell type in Alzheimer’s pathology. However, these cells play a double-edged role: some protect brain health, while others worsen neurodegeneration.
Understanding the functional differences between these microglial populations has been a research focus for Dr. Pinar Ayata, the study’s principal investigator and a professor with the CUNY ASRC neuroscience initiative and the CUNY Graduate Center’s Biology and Biochemistry programmes.
“We set out to answer what are the harmful microglia in Alzheimer’s disease and how can we therapeutically target them,” said Dr. Ayata. “We pinpointed a novel neurodegenerative microglia phenotype in Alzheimer’s disease characterised by a stress-related signalling pathway.”
The research team discovered that activation of this stress pathway, known as the integrated stress response (ISR), prompts microglia to produce and release toxic lipids. These lipids damage neurons and oligodendrocyte progenitor cells, two cell types that are essential for optimal brain function and that have most impact in Alzheimer’s disease. Blocking either the stress response, or the lipid synthesis pathway, reversed symptoms of Alzheimer’s disease in preclinical models.
Key findings
- Dark microglia and Alzheimer’s disease:
Using electron microscopy, the researchers identified an accumulation of 'dark microglia’ – a subset of microglia associated with cellular stress and neurodegeneration – in postmortem brain tissues from Alzheimer’s patients. These cells were present at twice the levels seen in healthy-aged individuals. - Toxic lipid secretion: The ISR pathway in microglia was shown to drive the synthesis and release of harmful lipids that contribute to synapse loss, a hallmark of Alzheimer’s disease.
- Therapeutic potential: In mouse models, inhibiting ISR activation or lipid synthesis prevented synapse loss and accumulation of neurodegenerative tau proteins, offering a promising pathway to explore for therapeutic intervention.
“These findings reveal a critical link between cellular stress and the neurotoxic effects of microglia in Alzheimer’s disease,” said Anna Flury, the study’s co-lead author and a team member at Ayata’s lab and a PhD student with the CUNY Graduate Center’s Biology programme.
“Targeting this pathway may open up new avenues for treatment by either halting the toxic lipid production or preventing the activation of harmful microglial phenotypes,” she added.
This research underscores the potential of developing drugs that target specific microglial populations or their stress-induced mechanisms. The study represents a major leap forward in understanding the cellular underpinnings of Alzheimer’s and emphasises the importance of microglial health in maintaining overall brain function.
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan


.jpg)














