-
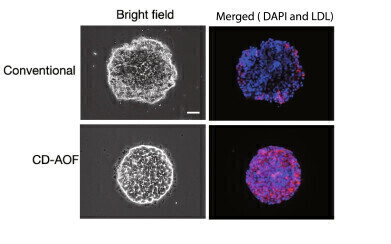 StemFit® for Differentiation in use: Lipid incorporation study in all iPSC-LBs by using DiI-Ac-LDL (Acetylated Low-Density Lipoprotein labelled with 1,1’-dioctadecyl-3,3,3’,3’- tetramethylindo-carbocyanine perchlorate). Scale bar, 50 µm. Figure adapted from Sekine, K., Ogawa, S., Tsuzuki, S., Kobayashi, T., Ikeda, K., Nakanishi, N., Takeuchi, K., Kanai, E., Otake, Y., Okamoto, S., Kobayashi, T., Takebe, T., & Taniguchi, H. (2020). Generation of human induced pluripotent stem cell-derived liver buds with chemically defined and animal origin-free media. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-73908-1
StemFit® for Differentiation in use: Lipid incorporation study in all iPSC-LBs by using DiI-Ac-LDL (Acetylated Low-Density Lipoprotein labelled with 1,1’-dioctadecyl-3,3,3’,3’- tetramethylindo-carbocyanine perchlorate). Scale bar, 50 µm. Figure adapted from Sekine, K., Ogawa, S., Tsuzuki, S., Kobayashi, T., Ikeda, K., Nakanishi, N., Takeuchi, K., Kanai, E., Otake, Y., Okamoto, S., Kobayashi, T., Takebe, T., & Taniguchi, H. (2020). Generation of human induced pluripotent stem cell-derived liver buds with chemically defined and animal origin-free media. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-73908-1 -
Reagents
GMP-grade stem cell media simplifies transition to clinical manufacturing
Mar 11 2025
AMSBIO has introduced GMP-grade StemFit® for Differentiation, a chemically defined, animal component-free medium designed for consistent and efficient differentiation of human induced pluripotent stem (hiPS) and embryonic stem (hES) cells. Now compliant with Good Manufacturing Practices (GMP), this formulation supports seamless progression to clinical applications.
By eliminating animal-derived components, StemFit® for Differentiation minimises immunogenic risks while ensuring lot-to-lot consistency. Manufactured under strict GMP guidelines, it provides a reliable solution for the reproducible expansion and differentiation of stem cell-derived tissues in clinical settings.
Dr Satoshi Okamoto of Yokohama City University Graduate School of Medicine noted:
"Using StemFit® Basic03 for iPSC culture alongside StemFit® for Differentiation, we established a stable, animal-origin-free protocol."
By the end of 2025, all StemFit® formulations, including Basic03 and Basic04 CT, along with key proteins such as bFGF and Activin A, will be available exclusively as GMP-grade versions. This transition enables labs to move from research to clinical manufacturing without the need to retest materials or adjust protocols, reducing both complexity and costs.
To make GMP adoption accessible, AMSBIO has optimised manufacturing to minimise cost differences between research and GMP-grade products
Aisha Amari, a stem cell specialist at AMSBIO, commented:
"With nearly 1,500 citations and growing use in cell and gene therapy, StemFit® has become a trusted solution for researchers and biotech companies worldwide."
Contact AMSBIO for further details about GMP compliant products and services.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh