-
 Bottle of StemFit Basic04 CT.
Bottle of StemFit Basic04 CT. -
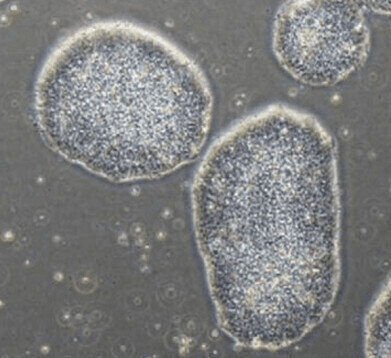 Colony of iPSCs grown using StemFit Basic04 CT.
Colony of iPSCs grown using StemFit Basic04 CT.
Reagents
GMP-compliant cell culture medium optimises API manufacture
Apr 30 2024
AMSBIO has unveiled an upgraded GMP-compliant version of its animal-origin-free, chemically defined StemFit™ iPSC expansion medium. StemFit™ Basic04 CT is engineered to enhance the growth and pluripotency of induced pluripotent stem cells (iPSC) while meeting the stringent Good Manufacturing Practice (GMP) standards for Active Pharmaceutical Ingredients (API).
With its superior iPSC performance, StemFit™ Basic04 CT facilitates expansion and sustains long-term genetic stability, optimising single-cell passaging for large-scale stem cell production. The manufacturing and quality control of this exciting new product are managed in strict accordance with GMP standards.
Each bottle of StemFit™ Basic04 CT is accompanied by a certificate of analysis and all necessary documentation, ensuring a seamless transition to clinical applications. Aisha Amari, Business Development specialist at AMSBIO, emphasised the significance of iPSCs in revolutionising cellular medicines and highlighted the pivotal role of GMP-compliant StemFit™ Basic04 CT in iPSC manufacturing for cell therapy.
Amari stated: "Using StemFit™, we hope researchers can further advance translational research, drug discovery and development and help bring high-quality iPSC based- therapies from bench to bedside."
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh