Mass spectrometry & spectroscopy
New Reaction Monitoring Solutions for benchtop NMR Spectrometer Released
Jan 13 2016
Magritek have released new solutions that enable straightforward reaction monitoring on the Spinsolve benchtop NMR spectrometer. This is complemented with a series of applications notes illustrating the multiple uses for the Spinsolve benchtop NMR spectrometer.
With high stability and spectral resolution Magritek’s Spinsolve delivers measurements that are quantitative with only minimal calibration. The signal has a linear response to concentration and it is generally not sensitive to the matrix. Results are representative of the complete sample and the measurement is non-destructive.
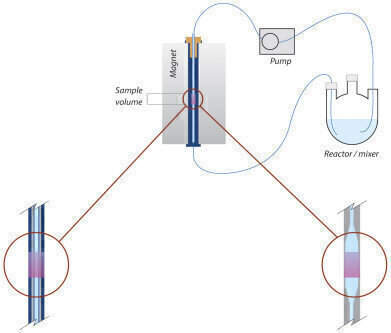
To enable the Spinsolve spectrometer for reaction monitoring Magritek have created full solutions with either glass flow cells or PTFE flow tubing that fit through the completely clear bore of the instrument. The glass flow cell option offers a large sample volume in the sampling region of the spectrometer and a narrow internal diameter outside of that zone. This gives the maximum signal to noise output with minimal fluid in the flow system. The hardware kit is completed with a peristaltic pump, appropriate tubing, and a stand to provide access below the spectrometer. Complementing the hardware is the full experiment scripting capability and pump control available in the latest instrument software. Stop-flow or continuous flow monitoring is straightforward to set up.
A range of application notes available on the Magritek website help Spinsolve users solve specific problems. For example, consider fermentation processes. This is a naturally occurring chemical event. One application note describes how easy it is to monitor a simple fermentation process - to follow the conversion of fructose and glucose into ethanol and carbon dioxide - and to easily get chemical conversion rates and to see where the optimal end point has occurred. The note shows that a simple flow loop setup allows on-line reaction monitoring on a lab bench. While shimming was performed at the start of the experiment on the reactant itself, no extra shimming was required during the ten day experiment. Even gas bubbles in the measurement volume did not disrupt subsequent measurements.
Another application note describes the study the hydrolysis of acetic anhydride by water. Rate constants can easily and reliably be obtained by plotting the peak height or integral of a characteristic peak as a function of reaction time. The activation energy of the process can be determined by carrying out the reaction at different temperatures.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh