Laboratory products
CE Mark Conformity for Cryogenic Vials
Oct 20 2010
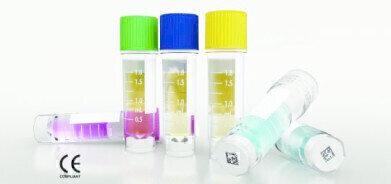
Wheaton Science Products has announced that its CryoELITE™ Cryogenic Vials are CE marked in accordance with the requirements of Directive 8/79/EC on in vitro diagnostic medical devices. The vials are available from laboratory distributors located throughout the European Economic Area (EEA).
The Wheaton CryoELITE line of polypropylene cryogenic vials is specifically designed for the failsafe cryopreservation of biospecimens for long-term storage and the efficient traceability for future retrieval. To ensure maintenance of sample integrity during low temperature storage and shipping, CryoELITE vials feature a vial and cap configuration that provides more surface contact that ensures an absolute seal to eliminate leakage that would contaminate the sample. The seal created by the unique manufacturing design exceeds DOT and IATA regulation standards by an average of 35% making them the superior choice for transport of valuable clinical samples as well.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh