Laboratory products
|
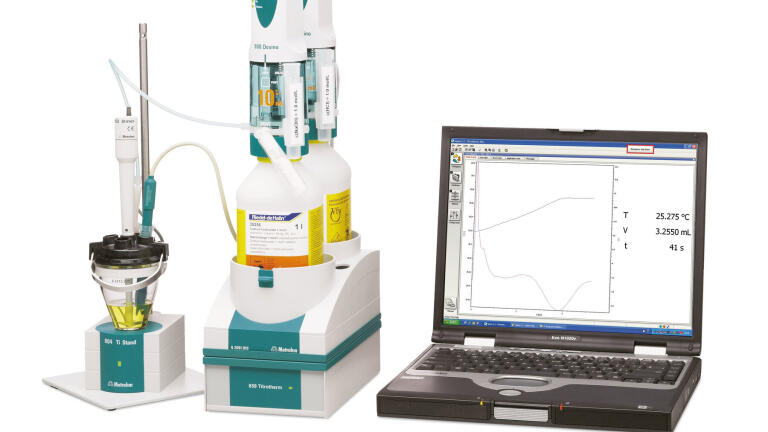
Weak organic bases soluble in nonaqueous solvents (including nonpolar solvents) may be determined by titration with strong acids such as anhydrous perchloric or trifluoromethanesulfonic acid in glacial acetic acid. The endpoint of such titrations may be determined thermometrically, if a suitable thermometric endpoint indicator is used. Isobutyl vinyl ether (IBVE) has been found to be very suitable for this purpose. Download Application Note H-129 for detailed information. |
|
|
The determination of weakly basic organic compounds soluble only in nonaqueous solvents, particularly nonpolar solvents, may present difficulties when employing titration techniques other than thermometric titrimetry. While enthalpies of neutralisation may be too low to obtain a satisfactory thermometric titration endpoint unaided, the use of a thermometric indicator enables very sharp endpoints to be obtained. Isobutyl vinyl ether (IBVE) has been found to be particularly suitable as such an indicator (Greenhow and Spencer, 1973). When all weak base has been neutralised by the titrant acid, the first trace of excess acid catalyses the strongly exothermic polymerisation of IBVE, thus marking the titration endpoint. Thermometric titration is faster than potentiometry (less than 3 minutes), there is no maintenance of the sensor required, and one and the same sensor can be used for all titrations (acid/base, redox, EDTA, precipitation, non-aqueous). Moreover, the sensor does not even have to be calibrated and it is completely insensitive to aggressive media. |